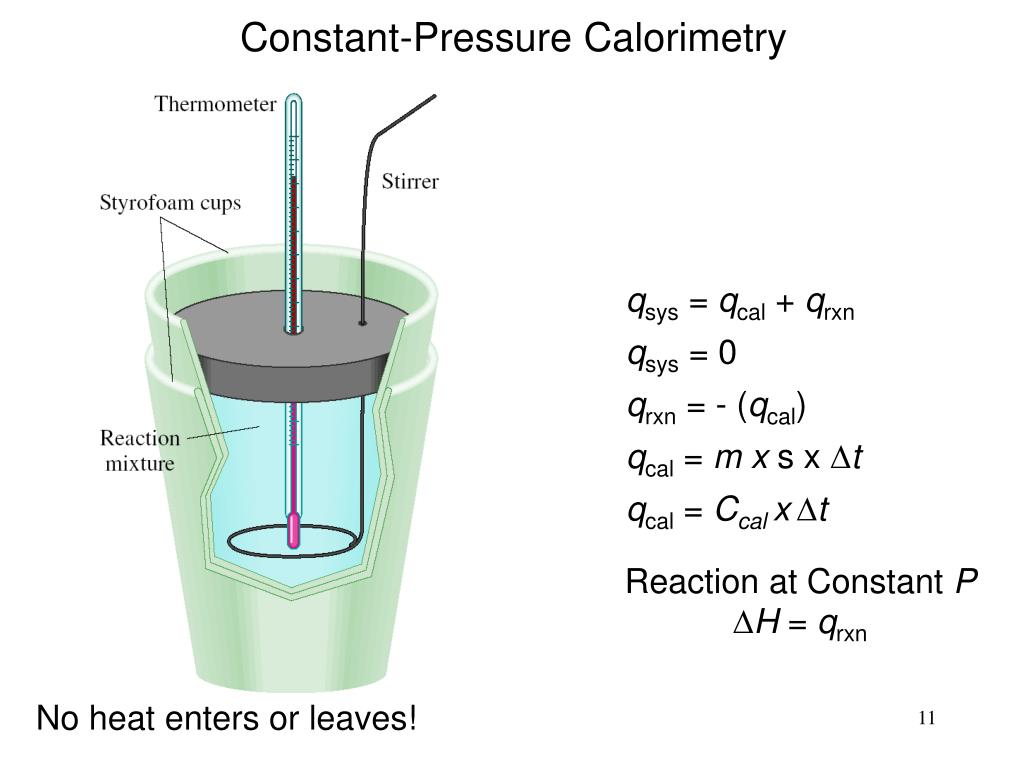
Principle Of Calorimetry . Compare heat flow from hot to cold objects in an ideal. Explore the principle, types, and applications of calorimetry. Heat transfer restores thermal equilibrium once the water and pan are in contact; The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. It stops once thermal equilibrium between the pan and the water is achieved. Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. It uses devices called calorimeters , which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The heat lost by the pan is. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. The body at higher temperature releases heat while the body at lower temperature absorbs heat. The principle of calorimetry indicates the law. For example, when an exothermic.
from www.slideserve.com
The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Explore the principle, types, and applications of calorimetry. The body at higher temperature releases heat while the body at lower temperature absorbs heat. It uses devices called calorimeters , which measure the change in. For example, when an exothermic. The heat lost by the pan is. Compare heat flow from hot to cold objects in an ideal. It stops once thermal equilibrium between the pan and the water is achieved. Heat transfer restores thermal equilibrium once the water and pan are in contact; A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
PPT Calorimetry PowerPoint Presentation, free download ID1875569
Principle Of Calorimetry The principle of calorimetry indicates the law. It uses devices called calorimeters , which measure the change in. It stops once thermal equilibrium between the pan and the water is achieved. The heat lost by the pan is. Compare heat flow from hot to cold objects in an ideal. Explore the principle, types, and applications of calorimetry. Apply the first law of thermodynamics to calorimetry. The principle of calorimetry indicates the law. For example, when an exothermic. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The body at higher temperature releases heat while the body at lower temperature absorbs heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat transfer restores thermal equilibrium once the water and pan are in contact; Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed.
From quizunderslung.z4.web.core.windows.net
What Is Direct Calorimetry Principle Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Heat transfer restores thermal equilibrium once the water and pan are in contact; The heat lost by the pan is. It stops once thermal equilibrium between the. Principle Of Calorimetry.
From www.researchgate.net
Basic principle of isothermal titration calorimetry. Schematic Principle Of Calorimetry Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters , which measure the change in. Apply the first law of thermodynamics to calorimetry. It stops once thermal equilibrium between the pan and the water is achieved. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement. Principle Of Calorimetry.
From www.youtube.com
CALORIMETRY Principle of Calorimetry, Calorimeter, it's application Principle Of Calorimetry Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. The body at higher temperature releases heat while the body at lower temperature absorbs heat. For example, when an exothermic. Explore the principle, types, and applications of calorimetry. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that. Principle Of Calorimetry.
From saylordotorg.github.io
Calorimetry Principle Of Calorimetry The heat lost by the pan is. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The principle of calorimetry indicates the law. Heat transfer restores thermal equilibrium once the water and pan are in contact; It stops once thermal equilibrium between the pan and the water is achieved. Compare heat flow from hot to. Principle Of Calorimetry.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Principle Of Calorimetry Heat transfer restores thermal equilibrium once the water and pan are in contact; A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The body at higher temperature releases heat while the body at lower temperature absorbs heat. Compare heat flow from hot to cold objects in an ideal. Learn how. Principle Of Calorimetry.
From byjus.com
State the Principle of calorimetry Principle Of Calorimetry Compare heat flow from hot to cold objects in an ideal. The body at higher temperature releases heat while the body at lower temperature absorbs heat. Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. Heat transfer restores thermal equilibrium once the water and pan are in contact; Explore the principle, types, and. Principle Of Calorimetry.
From www.slideserve.com
PPT ITC (Isothermal Titration Calorimetry) PowerPoint Presentation Principle Of Calorimetry Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters , which measure the change in. It stops once thermal equilibrium between the pan and the water is achieved. Apply the first law of thermodynamics to calorimetry. The heat lost by the pan is. Learn how to measure heat exchange and. Principle Of Calorimetry.
From users.highland.edu
Calorimetry Principle Of Calorimetry Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explore the principle, types, and applications of calorimetry. The principle of calorimetry indicates the law. The body at higher temperature releases heat while the body. Principle Of Calorimetry.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Principle Of Calorimetry It uses devices called calorimeters , which measure the change in. It stops once thermal equilibrium between the pan and the water is achieved. Compare heat flow from hot to cold objects in an ideal. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. For example, when. Principle Of Calorimetry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1551165 Principle Of Calorimetry The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. It stops once thermal equilibrium between the pan and the water is achieved. The heat lost by the pan is. Apply the first law of thermodynamics to calorimetry. The principle of calorimetry indicates the law. For example, when. Principle Of Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Principle Of Calorimetry The body at higher temperature releases heat while the body at lower temperature absorbs heat. Compare heat flow from hot to cold objects in an ideal. The heat lost by the pan is. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. A calorimeter is a device. Principle Of Calorimetry.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Principle Of Calorimetry Explore the principle, types, and applications of calorimetry. Apply the first law of thermodynamics to calorimetry. It stops once thermal equilibrium between the pan and the water is achieved. Heat transfer restores thermal equilibrium once the water and pan are in contact; Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. Calorimetry is. Principle Of Calorimetry.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Principle Of Calorimetry The heat lost by the pan is. For example, when an exothermic. Explore the principle, types, and applications of calorimetry. Heat transfer restores thermal equilibrium once the water and pan are in contact; It uses devices called calorimeters , which measure the change in. Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science.. Principle Of Calorimetry.
From www.slideserve.com
PPT Thermochemistry Notes PowerPoint Presentation, free download ID Principle Of Calorimetry It uses devices called calorimeters , which measure the change in. It stops once thermal equilibrium between the pan and the water is achieved. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Compare heat flow from hot to cold objects in. Principle Of Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry I Principle Of Calorimetry Compare heat flow from hot to cold objects in an ideal. It uses devices called calorimeters , which measure the change in. For example, when an exothermic. Heat transfer restores thermal equilibrium once the water and pan are in contact; Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Explore the principle, types, and applications. Principle Of Calorimetry.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Principle Of Calorimetry Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. It stops once thermal equilibrium between the pan and the water is achieved. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The body at higher temperature releases heat while the body at lower temperature absorbs heat. Heat transfer. Principle Of Calorimetry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Principle Of Calorimetry It stops once thermal equilibrium between the pan and the water is achieved. Compare heat flow from hot to cold objects in an ideal. Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. For example, when an exothermic. The body at higher temperature releases heat while the body at lower temperature absorbs heat.. Principle Of Calorimetry.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Principle Of Calorimetry The principle of calorimetry indicates the law. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Principle Of Calorimetry.
From themasterchemistry.com
Calorimeter Types, Principle, Working, Uses Principle Of Calorimetry The body at higher temperature releases heat while the body at lower temperature absorbs heat. For example, when an exothermic. The heat lost by the pan is. Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. The principle of calorimetry indicates the law. It stops once thermal equilibrium between the pan and the. Principle Of Calorimetry.
From www.researchgate.net
Principle of calorimetry. Download Scientific Diagram Principle Of Calorimetry For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The heat lost by the pan is. It uses devices called calorimeters , which measure the change in. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Explore the principle, types,. Principle Of Calorimetry.
From www.studypool.com
SOLUTION Calorimeter and principle of calorimetry Studypool Principle Of Calorimetry The body at higher temperature releases heat while the body at lower temperature absorbs heat. Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. For example, when an exothermic. It stops once thermal equilibrium between the pan and the water is achieved. Heat transfer restores thermal equilibrium once the water and pan are. Principle Of Calorimetry.
From www.youtube.com
Calorimetry Principle of Calorimetry Class 11NEET IIT Physics Principle Of Calorimetry The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Heat transfer restores thermal equilibrium once the water and pan are in contact; The principle of calorimetry indicates the law. It uses devices called calorimeters , which measure the change in. A calorimeter is a device used to. Principle Of Calorimetry.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure Principle Of Calorimetry Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The heat lost by the pan is. It stops once thermal equilibrium between the pan and the water is achieved. Compare heat flow from hot to cold. Principle Of Calorimetry.
From www.thoughtco.com
Calorimeter Definition in Chemistry Principle Of Calorimetry Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. For example, when an exothermic. The heat lost by the pan is. The principle of calorimetry indicates the law.. Principle Of Calorimetry.
From www.youtube.com
Principle of calorimetry ,Latent heat , Graphical representation of Principle Of Calorimetry The body at higher temperature releases heat while the body at lower temperature absorbs heat. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. For example, when an exothermic. Compare heat flow from hot to cold objects in an ideal. It uses devices called calorimeters , which measure the change in. The heat lost by. Principle Of Calorimetry.
From courses.lumenlearning.com
5.2 Calorimetry Chemistry Principle Of Calorimetry Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The principle of calorimetry indicates the law. Explore the principle, types, and applications of calorimetry. Calorimetry is the set of techniques used to measure enthalpy. Principle Of Calorimetry.
From www.researchgate.net
The principles and practice of isothermal titration calorimetry Principle Of Calorimetry Apply the first law of thermodynamics to calorimetry. It uses devices called calorimeters , which measure the change in. Compare heat flow from hot to cold objects in an ideal. Heat transfer restores thermal equilibrium once the water and pan are in contact; Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Learn how to. Principle Of Calorimetry.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Principle Of Calorimetry A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Apply the first law of thermodynamics to calorimetry. Explore the principle, types, and applications of calorimetry. The heat lost by the pan is. The body at higher temperature releases heat while the body at lower temperature absorbs heat. Compare heat flow. Principle Of Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Principle Of Calorimetry The body at higher temperature releases heat while the body at lower temperature absorbs heat. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. The heat lost by the pan is. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Compare heat. Principle Of Calorimetry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Principle Of Calorimetry Heat transfer restores thermal equilibrium once the water and pan are in contact; The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. For example, when an exothermic. The principle of calorimetry indicates the law. Explore the principle, types, and applications of calorimetry. Compare heat flow from hot. Principle Of Calorimetry.
From www.slideserve.com
PPT Energy Changes in Reactions PowerPoint Presentation, free Principle Of Calorimetry Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Explore the principle, types, and applications of calorimetry. The principle of calorimetry indicates the law. It stops once thermal equilibrium between the pan and the. Principle Of Calorimetry.
From fyohqoqgp.blob.core.windows.net
Calorimeter Formula In Physics at Caroline Graig blog Principle Of Calorimetry Heat transfer restores thermal equilibrium once the water and pan are in contact; Learn how to measure heat exchange and calculate enthalpy change using calorimetry, a branch of science. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It stops once thermal equilibrium between the pan and the water is achieved. Explore the principle, types,. Principle Of Calorimetry.
From www.youtube.com
Ap Physics Principle Of Calorimetry Heat & Temperature YouTube Principle Of Calorimetry The heat lost by the pan is. Heat transfer restores thermal equilibrium once the water and pan are in contact; The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. The body at higher temperature releases heat while the body at lower temperature absorbs heat. A calorimeter is. Principle Of Calorimetry.
From faqguide.co
What does a calorimeter do? Explained by FAQGuide Principle Of Calorimetry It stops once thermal equilibrium between the pan and the water is achieved. It uses devices called calorimeters , which measure the change in. The body at higher temperature releases heat while the body at lower temperature absorbs heat. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat. Principle Of Calorimetry.
From www.youtube.com
Principle of Calorimetry YouTube Principle Of Calorimetry Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The principle of calorimetry signifies the “law of conservation of energy.” hence, this statement means that the total amount of heat absorbed. Apply the first law of thermodynamics to calorimetry. It uses devices called calorimeters , which measure the change in. A calorimeter is a device. Principle Of Calorimetry.