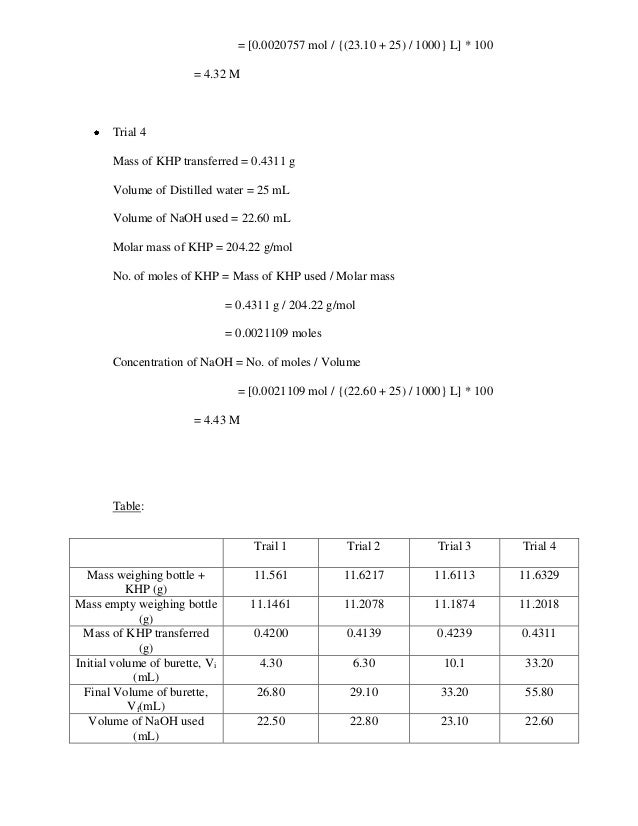
Acid And Base Lab Report . Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. Introduction to acids base chemistry part a experimental determination of acid dissociation constant, ka. Be able to determine the k a or k b from ph data associated with the titration. we will see how the acidity of substances is measured; to determine the ph of common solutions. Conversely, a base is a compound that accepts a hydrogen ion in a reaction. To understand ph differences of acids and bases. When the acid and base react,. The experiment was started by taking 1. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. an acid is a compound that supplies a hydrogen ion in a reaction.
from www.slideshare.net
The experiment was started by taking 1. Introduction to acids base chemistry part a experimental determination of acid dissociation constant, ka. an acid is a compound that supplies a hydrogen ion in a reaction. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Be able to determine the k a or k b from ph data associated with the titration. to determine the ph of common solutions. we will see how the acidity of substances is measured; the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions.
Chemistry Lab Report on standardization of acid and bases.
Acid And Base Lab Report to determine the ph of common solutions. to determine the ph of common solutions. When the acid and base react,. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). To understand ph differences of acids and bases. the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. Be able to determine the k a or k b from ph data associated with the titration. Conversely, a base is a compound that accepts a hydrogen ion in a reaction. Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. we will see how the acidity of substances is measured; The experiment was started by taking 1. Introduction to acids base chemistry part a experimental determination of acid dissociation constant, ka. acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. an acid is a compound that supplies a hydrogen ion in a reaction.
From www.studypool.com
SOLUTION CHM150 Phoenix WK3 AcidBase Chemistry Lab Report Studypool Acid And Base Lab Report the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. an acid is a compound that supplies a hydrogen ion in a reaction. Introduction to acids base chemistry part a experimental determination of acid dissociation constant, ka. Be able to determine the k a or k b from ph. Acid And Base Lab Report.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Acid And Base Lab Report Conversely, a base is a compound that accepts a hydrogen ion in a reaction. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). When the acid and base react,. Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. an. Acid And Base Lab Report.
From paperap.com
Acid And Base Lab Report Free Essay Example Acid And Base Lab Report to determine the ph of common solutions. The experiment was started by taking 1. Be able to determine the k a or k b from ph data associated with the titration. When the acid and base react,. we will see how the acidity of substances is measured; Using the reaction with hydrochloric acid in water as an example. Acid And Base Lab Report.
From www.scribd.com
Lab AcidBase Objectives Acid Dissociation (Chemistry) Free 30 Acid And Base Lab Report acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. Be able to determine the k a or k b from ph data associated with the titration. Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. When the acid. Acid And Base Lab Report.
From www.chegg.com
REPORT SHEET LAB AcidBase Titration 12 A. Concent... Acid And Base Lab Report Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. an acid is a compound that supplies a hydrogen ion in a reaction. The experiment was started by. Acid And Base Lab Report.
From haileeqohicks.blogspot.com
Acid Base Titration Experiment Report HaileeqoHicks Acid And Base Lab Report to determine the ph of common solutions. acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. Using the reaction with hydrochloric acid in water as an example to demonstrate. Acid And Base Lab Report.
From aylinmeowstuart.blogspot.com
Acid Base Titration Experiment Report Acid And Base Lab Report the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. Introduction to acids base chemistry part a experimental determination of acid dissociation constant, ka. Conversely, a base is a. Acid And Base Lab Report.
From www.studeersnel.nl
Acids and bases laboratory report Repeat the procedure twice more Acid And Base Lab Report Be able to determine the k a or k b from ph data associated with the titration. acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. an acid is a compound that supplies a hydrogen ion in a reaction. the purpose of this particular lab experiment was to. Acid And Base Lab Report.
From www.studocu.com
Lab9Lab ReportAcids and bases Lab 9 ChemcollectiveAcid and Base Acid And Base Lab Report acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Conversely, a base is a compound that accepts a hydrogen ion in a reaction. Introduction to acids base chemistry part a experimental determination of. Acid And Base Lab Report.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Acid And Base Lab Report Be able to determine the k a or k b from ph data associated with the titration. Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. Conversely, a base is a compound that accepts a hydrogen ion in a reaction. the purpose of this particular lab experiment. Acid And Base Lab Report.
From www.chegg.com
Solved as a Acids and Bases Lab report 1. Complete Arrhenius Acid And Base Lab Report acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. Be able to determine the k a or k b from ph data associated with the titration. Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. we will. Acid And Base Lab Report.
From www.studocu.com
LAB Report AcidsAND Bases TITLE Acids and Bases OBJECTIVE The Acid And Base Lab Report When the acid and base react,. an acid is a compound that supplies a hydrogen ion in a reaction. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. we. Acid And Base Lab Report.
From www.studypool.com
SOLUTION CHM150 Phoenix WK3 AcidBase Chemistry Lab Report Studypool Acid And Base Lab Report the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. to determine the ph of common solutions. acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. When the acid and base react,. Using the reaction with hydrochloric acid in. Acid And Base Lab Report.
From www.coursehero.com
[Solved] Lab report on Acid base titration Course Hero Acid And Base Lab Report acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. an acid is a compound that supplies a hydrogen ion in a reaction. Conversely, a base is a compound. Acid And Base Lab Report.
From studyhippo.com
This is a chemistry lab report on an AcidBase Essay Example Acid And Base Lab Report Be able to determine the k a or k b from ph data associated with the titration. When the acid and base react,. The experiment was started by taking 1. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). the purpose of this particular lab experiment was to effectively use titration. Acid And Base Lab Report.
From www.studocu.com
Lab Report 14 Acids, Bases, Salts and Buffers "Acids and bases are Acid And Base Lab Report Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. The experiment was started by taking 1. the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. Be able to determine the k a or k b from ph. Acid And Base Lab Report.
From www.studocu.com
Acids and Bases Lab MT Virtual Lab Report Acids and Bases Acidity Acid And Base Lab Report To understand ph differences of acids and bases. we will see how the acidity of substances is measured; the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. Be able to determine the k a or k b from ph data associated with the titration. in equation 1,. Acid And Base Lab Report.
From www.scribd.com
acidbase lab report Titration Hydrochloric Acid Acid And Base Lab Report Be able to determine the k a or k b from ph data associated with the titration. to determine the ph of common solutions. an acid is a compound that supplies a hydrogen ion in a reaction. we will see how the acidity of substances is measured; The experiment was started by taking 1. To understand ph. Acid And Base Lab Report.
From willtemccarthy.blogspot.com
Acid Base Titration Experiment Report WillteMccarthy Acid And Base Lab Report When the acid and base react,. the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. The experiment was started by taking 1. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). an acid is a compound that supplies a hydrogen. Acid And Base Lab Report.
From exoimpeyf.blob.core.windows.net
Properties And Reactions Of Acids And Bases Lab Report at Raymond Acid And Base Lab Report When the acid and base react,. Introduction to acids base chemistry part a experimental determination of acid dissociation constant, ka. the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a. Acid And Base Lab Report.
From webapi.bu.edu
Discussion acid base titration lab report. Acid Base Titration Lab Acid And Base Lab Report an acid is a compound that supplies a hydrogen ion in a reaction. to determine the ph of common solutions. The experiment was started by taking 1. To understand ph differences of acids and bases. we will see how the acidity of substances is measured; in equation 1, the acid is hcl (hydrochloric acid) and the. Acid And Base Lab Report.
From davidswart.org
Acid and Base Lab with Report The Art of Science Acid And Base Lab Report Conversely, a base is a compound that accepts a hydrogen ion in a reaction. Be able to determine the k a or k b from ph data associated with the titration. acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. When the acid and base react,. we will see. Acid And Base Lab Report.
From www.slideshare.net
Chemistry Lab Report on standardization of acid and bases. Acid And Base Lab Report The experiment was started by taking 1. we will see how the acidity of substances is measured; acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. Be able. Acid And Base Lab Report.
From www.studocu.com
LAB Report Experiment 6 ACID AND BASE LAB REPORT CHM420 GENERAL Acid And Base Lab Report When the acid and base react,. Be able to determine the k a or k b from ph data associated with the titration. The experiment was started by taking 1. To understand ph differences of acids and bases. we will see how the acidity of substances is measured; Introduction to acids base chemistry part a experimental determination of acid. Acid And Base Lab Report.
From www.slideshare.net
Chemistry Lab Report on standardization of acid and bases. Acid And Base Lab Report Conversely, a base is a compound that accepts a hydrogen ion in a reaction. an acid is a compound that supplies a hydrogen ion in a reaction. the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. Introduction to acids base chemistry part a experimental determination of acid dissociation. Acid And Base Lab Report.
From www.studocu.com
Chem 106 lab report 14 Experiment 14 Acids, Bases, Salts, and Acid And Base Lab Report acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. we will see how the acidity of substances is measured; To understand ph differences of acids and bases. the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. Conversely, a. Acid And Base Lab Report.
From momentumclubs.org
😊 Acid base titration lab report conclusion. Sample Lab Report. 20190125 Acid And Base Lab Report acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. Conversely, a base is a compound that accepts a hydrogen ion in a reaction. to determine the ph of common solutions. an acid is a compound that supplies a hydrogen ion in a reaction. Introduction to acids base chemistry. Acid And Base Lab Report.
From www.scribd.com
Lab Report Acid Base Titration Acid Acid And Base Lab Report Conversely, a base is a compound that accepts a hydrogen ion in a reaction. Introduction to acids base chemistry part a experimental determination of acid dissociation constant, ka. acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. the purpose of this particular lab experiment was to effectively use titration. Acid And Base Lab Report.
From www.studypool.com
SOLUTION CHM 113 Experiment 8 Acid Base Titration Lab Report Studypool Acid And Base Lab Report we will see how the acidity of substances is measured; Introduction to acids base chemistry part a experimental determination of acid dissociation constant, ka. To understand ph differences of acids and bases. Be able to determine the k a or k b from ph data associated with the titration. in equation 1, the acid is hcl (hydrochloric acid). Acid And Base Lab Report.
From exoimpeyf.blob.core.windows.net
Properties And Reactions Of Acids And Bases Lab Report at Raymond Acid And Base Lab Report to determine the ph of common solutions. When the acid and base react,. Be able to determine the k a or k b from ph data associated with the titration. an acid is a compound that supplies a hydrogen ion in a reaction. the purpose of this particular lab experiment was to effectively use titration by initially. Acid And Base Lab Report.
From childhealthpolicy.vumc.org
😍 Titration of acids and bases lab report. Acid and Base Titrations Lab Acid And Base Lab Report Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. To understand ph differences of acids and bases. an acid is a compound that supplies a hydrogen ion in. Acid And Base Lab Report.
From childhealthpolicy.vumc.org
😍 Titration of acids and bases lab report. Acid and Base Titrations Lab Acid And Base Lab Report an acid is a compound that supplies a hydrogen ion in a reaction. To understand ph differences of acids and bases. Be able to determine the k a or k b from ph data associated with the titration. Conversely, a base is a compound that accepts a hydrogen ion in a reaction. the purpose of this particular lab. Acid And Base Lab Report.
From www.studocu.com
Lab Report Acid and Bases 1 Copyright Labster ApS 2021 All Rights Acid And Base Lab Report we will see how the acidity of substances is measured; Using the reaction with hydrochloric acid in water as an example to demonstrate the definitions of an acid and a base. Conversely, a base is a compound that accepts a hydrogen ion in a reaction. When the acid and base react,. in equation 1, the acid is hcl. Acid And Base Lab Report.
From studylib.net
Acid Base Extraction Lab report Acid And Base Lab Report acidic liquids have a higher concentration of h+ ions, while bases have a lower concentration of h+ ions. the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. The experiment was started by taking 1. an acid is a compound that supplies a hydrogen ion in a reaction.. Acid And Base Lab Report.
From melanie-chapter.blogspot.com
Titration Of Acids And Bases Lab Report Experiment 20 Answers 45+ Pages Acid And Base Lab Report the purpose of this particular lab experiment was to effectively use titration by initially standardizing the base sodium hydroxide,. Be able to determine the k a or k b from ph data associated with the titration. in equation 1, the acid is hcl (hydrochloric acid) and the base is naoh (sodium hydroxide). Introduction to acids base chemistry part. Acid And Base Lab Report.