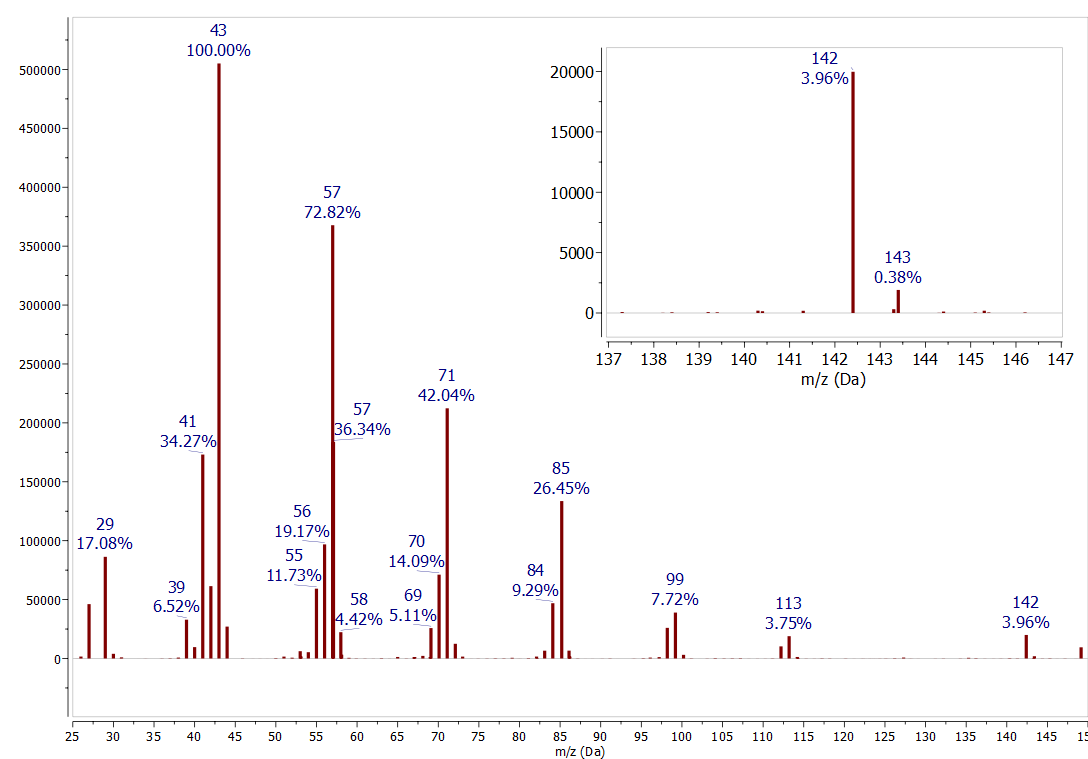
Peak Intensity Mass Spectrometry . This page describes how a mass spectrum is produced using a mass spectrometer. The peak that represents the unfragmented cation radical is called the. Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. An outline of what happens in a mass spectrometer. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. The tallest peak is called the base peak and is assigned 100% intensity. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the.
from chem.libretexts.org
Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. The peak that represents the unfragmented cation radical is called the. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. An outline of what happens in a mass spectrometer. The tallest peak is called the base peak and is assigned 100% intensity. This page describes how a mass spectrum is produced using a mass spectrometer.
6.4 Isotope Abundance Chemistry LibreTexts
Peak Intensity Mass Spectrometry The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. The tallest peak is called the base peak and is assigned 100% intensity. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. The peak that represents the unfragmented cation radical is called the. This page describes how a mass spectrum is produced using a mass spectrometer. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. An outline of what happens in a mass spectrometer.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation, free download ID5191047 Peak Intensity Mass Spectrometry An outline of what happens in a mass spectrometer. The tallest peak is called the base peak and is assigned 100% intensity. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. The peak that represents the unfragmented cation radical is called the. Most of the ions formed in a mass. Peak Intensity Mass Spectrometry.
From www.researchgate.net
Peakpicking for a single mass spectrum. Example of the peakdetection Peak Intensity Mass Spectrometry Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. The peak that represents the unfragmented cation radical is called. Peak Intensity Mass Spectrometry.
From www.researchgate.net
a) The peak intensity values for light and heavy CAP were observed with Peak Intensity Mass Spectrometry Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. The tallest peak is called the base peak and is. Peak Intensity Mass Spectrometry.
From blog.sepscience.com
The Role of Isotope Peak Intensities Obtained Using Mass Spectrometry Peak Intensity Mass Spectrometry This page describes how a mass spectrum is produced using a mass spectrometer. Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. The peak that represents the unfragmented cation radical is called the. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. Predict the relative heights. Peak Intensity Mass Spectrometry.
From www.instantuition.com
Mass Spectrometry of Chlorine O Level Chemistry Peak Intensity Mass Spectrometry This page describes how a mass spectrum is produced using a mass spectrometer. The peak that represents the unfragmented cation radical is called the. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. The tallest peak is. Peak Intensity Mass Spectrometry.
From www.youtube.com
Mass spectrometryMass spectroscopyMass spectrometerPrinciple Peak Intensity Mass Spectrometry Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. An outline of what happens in a mass spectrometer. Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. This. Peak Intensity Mass Spectrometry.
From mmerevise.co.uk
The Mass Spectrum & Relative Atomic Mass MME Peak Intensity Mass Spectrometry Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. Peak intensity directly reflects ionic. Peak Intensity Mass Spectrometry.
From www.researchgate.net
A plot of the total intensity ratio of m/z 109 m/z 108 peaks versus Peak Intensity Mass Spectrometry Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. This page describes how a mass spectrum is produced using a mass spectrometer. An outline of what happens in a mass spectrometer. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. The. Peak Intensity Mass Spectrometry.
From www.researchgate.net
Intensity fading mass spectrometry (MS) in model mixtures.(a Peak Intensity Mass Spectrometry The peak that represents the unfragmented cation radical is called the. An outline of what happens in a mass spectrometer. Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. This page describes how a mass spectrum is produced using a mass spectrometer. The tallest peak is called the base peak and is assigned 100% intensity. Most. Peak Intensity Mass Spectrometry.
From www.researchgate.net
Differential intensity of prothymosinα peak. SELDI mass spectrometry Peak Intensity Mass Spectrometry Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. An outline of what happens in a mass spectrometer. The most intense ion is assigned an abundance of 100, and it is referred. Peak Intensity Mass Spectrometry.
From www.researchgate.net
Reproducibility of LCMS analysis. (a) Base peak chromatogram (5080 Peak Intensity Mass Spectrometry The tallest peak is called the base peak and is assigned 100% intensity. This page describes how a mass spectrum is produced using a mass spectrometer. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. Predict the. Peak Intensity Mass Spectrometry.
From www.jeolusa.com
Mass Spectrometry Basics Mass Spectrometry JEOL USA Peak Intensity Mass Spectrometry Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. This page describes how a mass spectrum is produced using a mass spectrometer. An outline of what happens in a mass spectrometer. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance. Peak Intensity Mass Spectrometry.
From agora.cs.wcu.edu
Chromatography Peak Intensity Mass Spectrometry The peak that represents the unfragmented cation radical is called the. The tallest peak is called the base peak and is assigned 100% intensity. An outline of what happens in a mass spectrometer. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. This page describes how a mass spectrum is. Peak Intensity Mass Spectrometry.
From chem.libretexts.org
4.3 Mass Spectrometry Chemistry LibreTexts Peak Intensity Mass Spectrometry The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. An outline of what happens in a mass spectrometer. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the. Peak Intensity Mass Spectrometry.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation, free download ID2090241 Peak Intensity Mass Spectrometry The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. This page describes how a mass spectrum is produced using a mass spectrometer. An outline of what happens in a mass spectrometer. The tallest peak is called the base peak and is assigned 100% intensity. Peak intensity directly reflects ionic species. Peak Intensity Mass Spectrometry.
From alchetron.com
Mass spectrum Alchetron, The Free Social Encyclopedia Peak Intensity Mass Spectrometry Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. The peak that represents the unfragmented cation radical is called. Peak Intensity Mass Spectrometry.
From www.pinterest.com
Khan Academy Mass spectrometry, Spectrometers, Physics and mathematics Peak Intensity Mass Spectrometry The peak that represents the unfragmented cation radical is called the. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. An outline of what happens in a mass spectrometer. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. This page describes. Peak Intensity Mass Spectrometry.
From www.researchgate.net
Mass spectrometry (MS), analytical ultracentrifugation (AUC) and Peak Intensity Mass Spectrometry The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. This page describes how a mass spectrum is produced using a mass spectrometer. An outline of what happens in a mass spectrometer. The peak that represents the unfragmented cation radical is called the. Most of the ions formed in a mass. Peak Intensity Mass Spectrometry.
From study.com
How to Identify an Element from Its Mass Spectrum Chemistry Peak Intensity Mass Spectrometry The peak that represents the unfragmented cation radical is called the. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon. Peak Intensity Mass Spectrometry.
From www.youtube.com
Finding the molecular formula from a mass spectrum YouTube Peak Intensity Mass Spectrometry The peak that represents the unfragmented cation radical is called the. An outline of what happens in a mass spectrometer. This page describes how a mass spectrum is produced using a mass spectrometer. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. The tallest peak is called the base peak and. Peak Intensity Mass Spectrometry.
From chem.libretexts.org
6.4 Isotope Abundance Chemistry LibreTexts Peak Intensity Mass Spectrometry An outline of what happens in a mass spectrometer. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. The tallest peak is called the base peak and is assigned 100% intensity. Peak. Peak Intensity Mass Spectrometry.
From www.enovatia.com
Mass Accuracy and Resolution Novatia, LLC Peak Intensity Mass Spectrometry This page describes how a mass spectrum is produced using a mass spectrometer. The tallest peak is called the base peak and is assigned 100% intensity. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. An outline. Peak Intensity Mass Spectrometry.
From www.researchgate.net
Mass spectrometry data showing the stability of the compounds. ac Peak Intensity Mass Spectrometry Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. The peak that represents the unfragmented cation radical is called the. An outline of what happens in a mass spectrometer. The tallest peak is called the base peak and is assigned 100% intensity. The most intense ion is assigned an abundance of. Peak Intensity Mass Spectrometry.
From www.pinterest.com
Finding the molecular formula from a mass spectrum Molecular Peak Intensity Mass Spectrometry This page describes how a mass spectrum is produced using a mass spectrometer. The tallest peak is called the base peak and is assigned 100% intensity. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. The peak that represents the unfragmented cation radical is called the. Most of the ions. Peak Intensity Mass Spectrometry.
From mungfali.com
Mass Spectrometry Chart Peak Intensity Mass Spectrometry This page describes how a mass spectrum is produced using a mass spectrometer. The peak that represents the unfragmented cation radical is called the. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. The tallest peak is called the base peak and is assigned 100% intensity. Peak intensity directly reflects. Peak Intensity Mass Spectrometry.
From chem.libretexts.org
20.1 Molecular Mass Spectra Chemistry LibreTexts Peak Intensity Mass Spectrometry Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. Peak intensity directly reflects ionic. Peak Intensity Mass Spectrometry.
From ar.inspiredpencil.com
Gas Chromatography Mass Spectrometry Diagram Peak Intensity Mass Spectrometry The tallest peak is called the base peak and is assigned 100% intensity. The most intense ion is assigned an abundance of 100, and it is referred to as the base peak. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. The peak that represents the unfragmented cation radical is called. Peak Intensity Mass Spectrometry.
From www.youtube.com
Intensity Ratio of peaks in mass spectrum Mass Spectrometry Organic Peak Intensity Mass Spectrometry An outline of what happens in a mass spectrometer. Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. Most. Peak Intensity Mass Spectrometry.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation, free download ID4569410 Peak Intensity Mass Spectrometry Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. This page describes how a mass spectrum is produced using a mass spectrometer. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the. Peak Intensity Mass Spectrometry.
From www.chemistrystudent.com
Mass Spectrometry (ALevel) ChemistryStudent Peak Intensity Mass Spectrometry Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. The tallest peak is called the base peak and is assigned 100% intensity. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. The most intense ion is assigned an abundance of 100, and it is referred to as. Peak Intensity Mass Spectrometry.
From www.researchgate.net
Mass spectrometry on MbCD. Mass spectra of MbCD1,2, and3 show Peak Intensity Mass Spectrometry Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. An outline of what happens in a mass spectrometer. This page describes how a mass spectrum is produced using a mass spectrometer. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. The peak that represents the unfragmented cation. Peak Intensity Mass Spectrometry.
From www.youtube.com
Mass Spectrometry EDx Learning HSC Biology YouTube Peak Intensity Mass Spectrometry Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. The peak that represents the unfragmented cation radical is called the. An outline of what happens in a mass spectrometer. This page describes. Peak Intensity Mass Spectrometry.
From www.youtube.com
Mass Spectroscopy The Difference Between M+ (Parent) Peak vs Base Peak Peak Intensity Mass Spectrometry Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. The peak that represents the unfragmented cation radical is called the. The tallest peak is called the base peak and is assigned 100%. Peak Intensity Mass Spectrometry.
From www.researchgate.net
Is the most intense peak in my deconvoluted mass spec the peak I should Peak Intensity Mass Spectrometry Peak intensity directly reflects ionic species abundance with the respective m/z ratio [1]. Predict the relative heights of the m +·, (m + 1) +·, etc., peaks in the mass spectrum of a compound, given the natural abundance of the isotopes of carbon and the other elements present in the. The most intense ion is assigned an abundance of 100,. Peak Intensity Mass Spectrometry.
From www.tofwerk.com
Quantifying the Uncertainties of Fitted MS Peak Intensities TOFWERK Peak Intensity Mass Spectrometry This page describes how a mass spectrum is produced using a mass spectrometer. The peak that represents the unfragmented cation radical is called the. Most of the ions formed in a mass spectrometer have a single charge, so the m/z value is. An outline of what happens in a mass spectrometer. Predict the relative heights of the m +·, (m. Peak Intensity Mass Spectrometry.