What Is E In E Hv . $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. Where, e = energy of the photon. I have seen the energy of a photon given by the formulas: \small e = \frac {hc} {\lambda} = hf e = λhc = hf. How to calculate the energy of a photon. C = speed of light. E = hf or e = hc/λ. Planck's photo energy equation is: If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. This equation says that the energy of a.
from www.chegg.com
Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. I have seen the energy of a photon given by the formulas: Where, e = energy of the photon. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. This equation says that the energy of a. Planck's photo energy equation is: \small e = \frac {hc} {\lambda} = hf e = λhc = hf. C = speed of light.
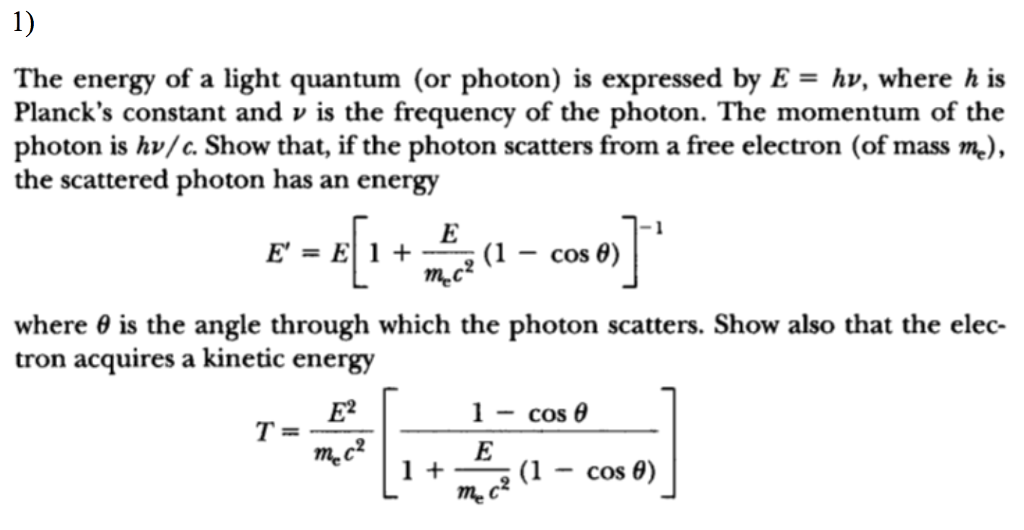
Solved The energy of a light quantum (or photon) is
What Is E In E Hv If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. \small e = \frac {hc} {\lambda} = hf e = λhc = hf. Where, e = energy of the photon. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? I have seen the energy of a photon given by the formulas: Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. How to calculate the energy of a photon. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. E = hf or e = hc/λ. C = speed of light. Planck's photo energy equation is: This equation says that the energy of a.
From mungfali.com
The Debroglie Wavelength Of An Electron Moving In The Nth Bohr Orbit 48D What Is E In E Hv A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? C = speed of light. E = hf or e. What Is E In E Hv.
From www.youtube.com
Physics 8.1.1.2 Solving problem involving the energy of a photon using What Is E In E Hv E = hf or e = hc/λ. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. Where, e = energy of the photon. How to calculate the energy of a photon. $$e = h. What Is E In E Hv.
From www.slideserve.com
PPT THERMODYNAMICS PowerPoint Presentation, free download ID6089405 What Is E In E Hv Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. Planck's photo energy equation is: This equation says that the energy of a. C = speed of light. How to calculate the energy of a photon. If a proton starts at frequency v, and its frequency increases, does its energy increase. What Is E In E Hv.
From socratic.org
What is the approximate energy of a photon having a frequency of 4*10^7 What Is E In E Hv $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. C = speed of light. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. Planck's photo energy equation is: I have seen the energy of a photon given by the formulas: How to calculate the energy. What Is E In E Hv.
From www.doubtnut.com
In Planck's oscillator energy is given as E=(hv)/(exp((hv)/(Kt)1)) If What Is E In E Hv E = hf or e = hc/λ. How to calculate the energy of a photon. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. I have seen the energy of a photon given. What Is E In E Hv.
From rechschem.weebly.com
Spectrum What Is E In E Hv Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? C = speed of light. How. What Is E In E Hv.
From www.youtube.com
A song for the PlanckEinstein equation E=hv YouTube What Is E In E Hv \small e = \frac {hc} {\lambda} = hf e = λhc = hf. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. Where, e = energy of the photon. This equation says that the energy of a. How to calculate the energy of a photon. C = speed of light. Planck's. What Is E In E Hv.
From studylib.net
Planck's Equation E = hv What Is E In E Hv If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. $$e = h \cdot f \tag{1}$$. What Is E In E Hv.
From www.youtube.com
HighVoltageTestingHVTestingHighVoltageEngineeringHVETypesHigh What Is E In E Hv This equation says that the energy of a. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? I have seen the energy of a photon given by the formulas: E = hf or e = hc/λ. Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black. What Is E In E Hv.
From diagramliboriginariosmb1.z13.web.core.windows.net
What Is Considered Low Voltage Electrical What Is E In E Hv E = hf or e = hc/λ. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. I have seen the energy of a photon given by the formulas: Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. This equation says that. What Is E In E Hv.
From www.numerade.com
SOLVED Which equation was used by Albert Einstein to explain the What Is E In E Hv This equation says that the energy of a. Planck's photo energy equation is: \small e = \frac {hc} {\lambda} = hf e = λhc = hf. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? Where, e = energy of the photon. C = speed of light. E = hf or e. What Is E In E Hv.
From www.storyofmathematics.com
Using the two equations E=hv and c=lambda v derive an equation What Is E In E Hv $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. Where, e = energy of the photon. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. E = hf or e = hc/λ. If a proton starts at frequency v, and its frequency increases, does its. What Is E In E Hv.
From www.bartleby.com
Answered What is the frequency of light emitted… bartleby What Is E In E Hv $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. C = speed of light. How to calculate the energy of a photon. Planck's photo energy equation is: This equation says that the energy of a.. What Is E In E Hv.
From brainly.in
The energy of a photon is E = hv and the momentum of photon p = (h What Is E In E Hv Planck's photo energy equation is: If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? Where, e = energy of the photon. E = hf or e = hc/λ. How to calculate the energy of a photon. $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. A. What Is E In E Hv.
From www.researchgate.net
Plots of (hv) 2 versus hv (eV) for all the prepared samples. Download What Is E In E Hv A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? Planck's photo energy equation is: I have seen the energy of a photon given by the formulas: How to calculate the energy of a photon.. What Is E In E Hv.
From chemcats.blogspot.com
Chemistry Ch 4 Notes through E=hv; Ch 4 Practice Problems WS due for What Is E In E Hv This equation says that the energy of a. I have seen the energy of a photon given by the formulas: Where, e = energy of the photon. How to calculate the energy of a photon. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. Planck's photo energy equation is: C =. What Is E In E Hv.
From www.youtube.com
Types of Voltages in tamil Basic Electrical ELV, LV, MV, HV, EHV What Is E In E Hv Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. E = hf or e = hc/λ. I have seen the energy of a photon given by the formulas: If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? Where, e = energy of. What Is E In E Hv.
From www.slideserve.com
PPT Electron Arrangement PowerPoint Presentation, free download ID What Is E In E Hv $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? \small e = \frac {hc} {\lambda} = hf e = λhc = hf. Where, e = energy of the photon. This equation says that the energy of a.. What Is E In E Hv.
From studylib.net
E = hv What Is E In E Hv C = speed of light. E = hf or e = hc/λ. How to calculate the energy of a photon. This equation says that the energy of a. Planck's photo energy equation is: I have seen the energy of a photon given by the formulas: A photon is characterized by either a wavelength, denoted by λ or equivalently an energy,. What Is E In E Hv.
From blog.sciencenet.cn
科学网—[请教] 《物理学》里光子(光量子)能量 E=hν 精确成立的条件是什么? 杨正瓴的博文 What Is E In E Hv E = hf or e = hc/λ. Where, e = energy of the photon. Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. Planck's photo energy equation is: \small e = \frac. What Is E In E Hv.
From byjus.com
When photons of energy hv fall on an aluminium plate (of work function What Is E In E Hv Planck's photo energy equation is: \small e = \frac {hc} {\lambda} = hf e = λhc = hf. E = hf or e = hc/λ. Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. Where, e = energy of the photon. This equation says that the energy of a. If. What Is E In E Hv.
From www.youtube.com
Relationship of delta E with q and w YouTube What Is E In E Hv This equation says that the energy of a. $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. C = speed of light. \small e = \frac {hc} {\lambda} = hf e = λhc = hf. Where, e = energy of the photon. If a proton starts at frequency v, and its frequency increases, does. What Is E In E Hv.
From www.chegg.com
Solved E2 E2 E2 2 hoE hr hv hv hv 定委工 ho E1 E1 E (a) (b) What Is E In E Hv C = speed of light. \small e = \frac {hc} {\lambda} = hf e = λhc = hf. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. Planck’s equation describes the quantity of spectral. What Is E In E Hv.
From www.chegg.com
2. (a) Show that the spectral density of the emitted What Is E In E Hv C = speed of light. $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? Where, e = energy of. What Is E In E Hv.
From www.youtube.com
Using E = hc/λ to Calculate Energy of a Wave (Example 4) YouTube What Is E In E Hv Planck's photo energy equation is: A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. This equation says that the energy of a. I have seen the energy of a photon given by the formulas: \small e = \frac {hc} {\lambda} = hf e = λhc = hf. Planck’s equation describes the. What Is E In E Hv.
From www.doubtnut.com
Photon is quantum of radiation with energy E =hv where v is frequency What Is E In E Hv Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. E = hf or e = hc/λ. Planck's photo. What Is E In E Hv.
From syzygyastro.hubpages.com
How the Physics of can Generate Electricity HubPages What Is E In E Hv \small e = \frac {hc} {\lambda} = hf e = λhc = hf. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? C = speed of light. E = hf or e = hc/λ. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e.. What Is E In E Hv.
From www.youtube.com
Quantized Energy and Photons Chemistry Tutorial YouTube What Is E In E Hv How to calculate the energy of a photon. Where, e = energy of the photon. E = hf or e = hc/λ. Planck's photo energy equation is: A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. \small e = \frac {hc} {\lambda} = hf e = λhc = hf. $$e =. What Is E In E Hv.
From blog.naver.com
e=hv 네이버 블로그 What Is E In E Hv Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. This equation says that the energy of a. I have seen the energy of a photon given by the formulas: Planck's photo energy equation is: A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted. What Is E In E Hv.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Delta E What Is E In E Hv How to calculate the energy of a photon. I have seen the energy of a photon given by the formulas: This equation says that the energy of a. Where, e = energy of the photon. C = speed of light. Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. Planck's. What Is E In E Hv.
From www.vrogue.co
Klasifikasi Besar Tegangan Lv Low Voltage Mv Medium V vrogue.co What Is E In E Hv How to calculate the energy of a photon. \small e = \frac {hc} {\lambda} = hf e = λhc = hf. This equation says that the energy of a. I have seen the energy of a photon given by the formulas: Planck's photo energy equation is: Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by. What Is E In E Hv.
From www.chegg.com
Solved Match the unit to the symbol in the following What Is E In E Hv Planck's photo energy equation is: A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. Where, e =. What Is E In E Hv.
From www.chegg.com
Solved The energy of a light quantum (or photon) is What Is E In E Hv I have seen the energy of a photon given by the formulas: Where, e = energy of the photon. This equation says that the energy of a. A photon is characterized by either a wavelength, denoted by λ or equivalently an energy, denoted by e. Planck's photo energy equation is: If a proton starts at frequency v, and its frequency. What Is E In E Hv.
From www.slideshare.net
Light What Is E In E Hv E = hf or e = hc/λ. Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. Where, e = energy of the photon. \small e = \frac {hc} {\lambda} = hf e = λhc = hf. This equation says that the energy of a. Planck's photo energy equation is: I. What Is E In E Hv.
From brainly.in
Find the dimension of planks constant 'h' from the equation E=hv where What Is E In E Hv Planck’s equation describes the quantity of spectral radiance at a particular wavelength radiated by a black body in equilibrium. $$e = h \cdot f \tag{1}$$ where $e$ = energy of the photon, $h$ =. If a proton starts at frequency v, and its frequency increases, does its energy increase or decrease? E = hf or e = hc/λ. This equation. What Is E In E Hv.