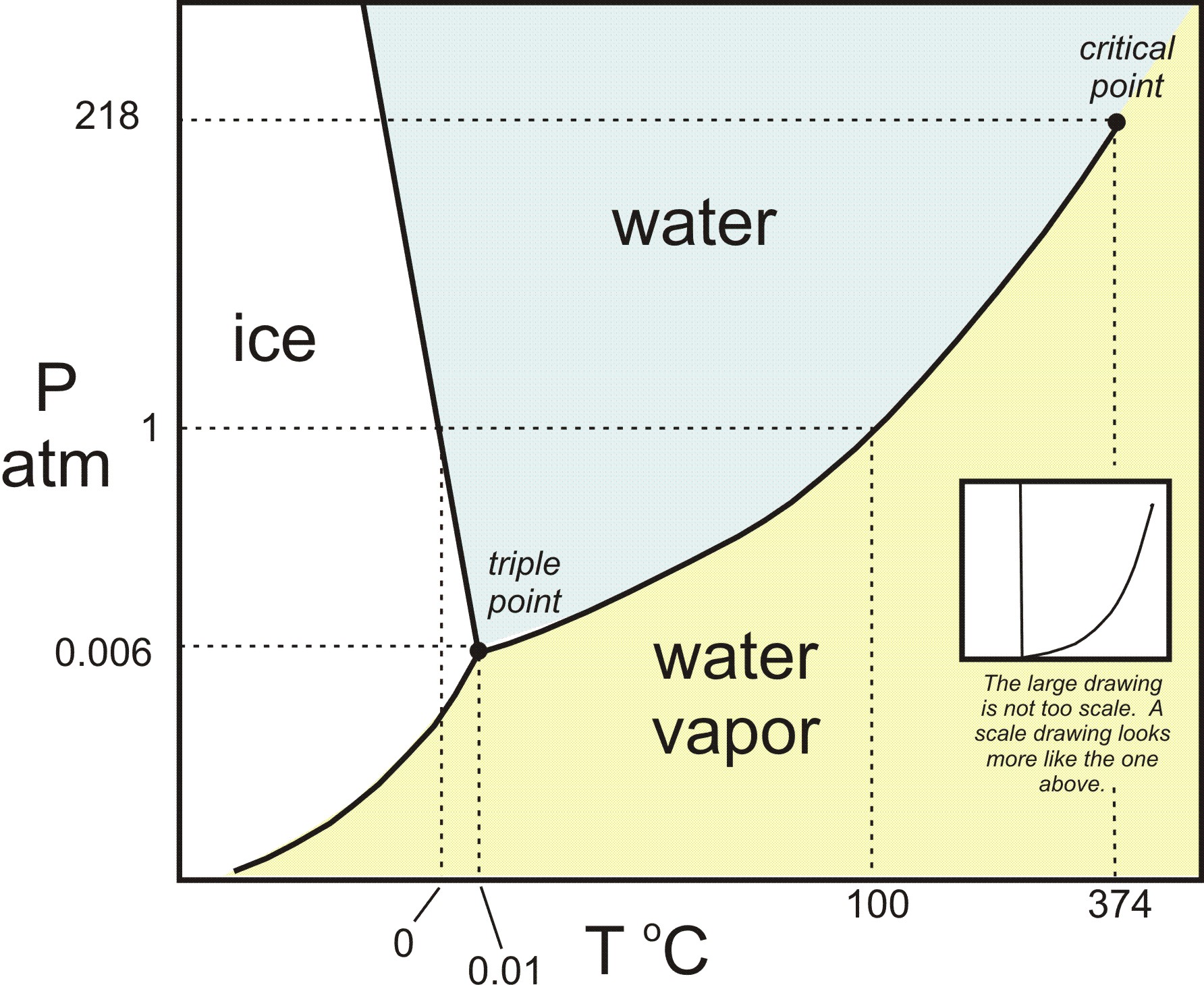
Why Do Solids And Liquids Coexist At Their Melting Point . The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. A phase transition like this, in which a solid compound changes into a liquid and a different solid, is called incongruent or. More heat then will convert the solid into a liquid with no temperature change. Assertion :the melting point is defined for solids when it converts from solid state to. Hence, solids and liquids co. As heat is applied to a solid, its temperature will increase until the melting point is reached. Greater the pressure, the lower the melting point. The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. At the melting point, the solid and liquid phase exist in: It is unique to a substance and is dependent on the pressure acting on the body. The temperature at which solid and liquid states coexist together is called the melting point.
from exyaeabov.blob.core.windows.net
Hence, solids and liquids co. At the melting point, the solid and liquid phase exist in: More heat then will convert the solid into a liquid with no temperature change. Assertion :the melting point is defined for solids when it converts from solid state to. It is unique to a substance and is dependent on the pressure acting on the body. The temperature at which solid and liquid states coexist together is called the melting point. As heat is applied to a solid, its temperature will increase until the melting point is reached. Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. Greater the pressure, the lower the melting point. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo).
What Is The Boiling Point Melting Point And Freezing Point Of Water at
Why Do Solids And Liquids Coexist At Their Melting Point Assertion :the melting point is defined for solids when it converts from solid state to. As heat is applied to a solid, its temperature will increase until the melting point is reached. Assertion :the melting point is defined for solids when it converts from solid state to. Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. Greater the pressure, the lower the melting point. Hence, solids and liquids co. More heat then will convert the solid into a liquid with no temperature change. It is unique to a substance and is dependent on the pressure acting on the body. At the melting point, the solid and liquid phase exist in: The temperature at which solid and liquid states coexist together is called the melting point. A phase transition like this, in which a solid compound changes into a liquid and a different solid, is called incongruent or. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo).
From studymind.co.uk
Changing State (GCSE Chemistry) Study Mind Why Do Solids And Liquids Coexist At Their Melting Point Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The temperature at which solid and liquid states coexist together is called the melting point. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). The pressure. Why Do Solids And Liquids Coexist At Their Melting Point.
From owlcation.com
What Are the Freezing, Melting, and Boiling Points of Solids, Liquids Why Do Solids And Liquids Coexist At Their Melting Point At the melting point, the solid and liquid phase exist in: Assertion :the melting point is defined for solids when it converts from solid state to. It is unique to a substance and is dependent on the pressure acting on the body. As heat is applied to a solid, its temperature will increase until the melting point is reached. The. Why Do Solids And Liquids Coexist At Their Melting Point.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Why Do Solids And Liquids Coexist At Their Melting Point The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). As heat is applied to a solid, its temperature will increase until the melting point is reached. The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. Melting. Why Do Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
Phase Changes. ppt download Why Do Solids And Liquids Coexist At Their Melting Point The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). A phase transition like this, in which a solid compound changes into a liquid and a different solid, is called incongruent or. At the melting point, the solid and liquid phase exist in: It is. Why Do Solids And Liquids Coexist At Their Melting Point.
From exymotkoo.blob.core.windows.net
Solid And Liquid Phases In Equilibrium at Jessica Florian blog Why Do Solids And Liquids Coexist At Their Melting Point It is unique to a substance and is dependent on the pressure acting on the body. More heat then will convert the solid into a liquid with no temperature change. Greater the pressure, the lower the melting point. A phase transition like this, in which a solid compound changes into a liquid and a different solid, is called incongruent or.. Why Do Solids And Liquids Coexist At Their Melting Point.
From exyaeabov.blob.core.windows.net
What Is The Boiling Point Melting Point And Freezing Point Of Water at Why Do Solids And Liquids Coexist At Their Melting Point As heat is applied to a solid, its temperature will increase until the melting point is reached. It is unique to a substance and is dependent on the pressure acting on the body. Hence, solids and liquids co. The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. At the melting point, the. Why Do Solids And Liquids Coexist At Their Melting Point.
From chem.libretexts.org
5.6 Phase Diagrams Chemistry LibreTexts Why Do Solids And Liquids Coexist At Their Melting Point Greater the pressure, the lower the melting point. A phase transition like this, in which a solid compound changes into a liquid and a different solid, is called incongruent or. At the melting point, the solid and liquid phase exist in: The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. Hence, solids. Why Do Solids And Liquids Coexist At Their Melting Point.
From exykjlgyk.blob.core.windows.net
Three Examples Each Of Solids Liquids And Gases at Meghan Anderson blog Why Do Solids And Liquids Coexist At Their Melting Point More heat then will convert the solid into a liquid with no temperature change. It is unique to a substance and is dependent on the pressure acting on the body. Hence, solids and liquids co. As heat is applied to a solid, its temperature will increase until the melting point is reached. Greater the pressure, the lower the melting point.. Why Do Solids And Liquids Coexist At Their Melting Point.
From unistudium.unipg.it
Phase Diagrams Why Do Solids And Liquids Coexist At Their Melting Point More heat then will convert the solid into a liquid with no temperature change. The temperature at which solid and liquid states coexist together is called the melting point. The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. Assertion :the melting point is defined for solids when it converts from solid state. Why Do Solids And Liquids Coexist At Their Melting Point.
From dxojwesjc.blob.core.windows.net
Lesson Plan Properties Of Solids Liquids And Gases at Albert Mitchell blog Why Do Solids And Liquids Coexist At Their Melting Point It is unique to a substance and is dependent on the pressure acting on the body. Hence, solids and liquids co. The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. A phase transition like this, in which a solid compound changes into a liquid and a different solid, is called incongruent or.. Why Do Solids And Liquids Coexist At Their Melting Point.
From exylluyva.blob.core.windows.net
Solid Liquid Gas Melting Point Boiling Point at Margaret Chaffins blog Why Do Solids And Liquids Coexist At Their Melting Point Greater the pressure, the lower the melting point. At the melting point, the solid and liquid phase exist in: As heat is applied to a solid, its temperature will increase until the melting point is reached. A phase transition like this, in which a solid compound changes into a liquid and a different solid, is called incongruent or. The melting. Why Do Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
MATTER & ITS PROPERTIES NOTESHEET ppt download Why Do Solids And Liquids Coexist At Their Melting Point More heat then will convert the solid into a liquid with no temperature change. At the melting point, the solid and liquid phase exist in: Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. A phase transition like this, in which a solid compound changes into a liquid and a different. Why Do Solids And Liquids Coexist At Their Melting Point.
From exylluyva.blob.core.windows.net
Solid Liquid Gas Melting Point Boiling Point at Margaret Chaffins blog Why Do Solids And Liquids Coexist At Their Melting Point Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. Hence, solids and liquids co. The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. More heat then will convert the solid into a liquid with no temperature change. It is unique to a substance. Why Do Solids And Liquids Coexist At Their Melting Point.
From owlcation.com
All About Matter An Introduction to the Basics Owlcation Why Do Solids And Liquids Coexist At Their Melting Point As heat is applied to a solid, its temperature will increase until the melting point is reached. Greater the pressure, the lower the melting point. More heat then will convert the solid into a liquid with no temperature change. Assertion :the melting point is defined for solids when it converts from solid state to. At the melting point, the solid. Why Do Solids And Liquids Coexist At Their Melting Point.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Why Do Solids And Liquids Coexist At Their Melting Point The temperature at which solid and liquid states coexist together is called the melting point. The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. Greater the pressure, the lower the melting point. At the melting point, the solid and liquid phase exist in: Assertion :the melting point is defined for solids when. Why Do Solids And Liquids Coexist At Their Melting Point.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Why Do Solids And Liquids Coexist At Their Melting Point Hence, solids and liquids co. Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). A phase transition like this, in which a solid compound changes into. Why Do Solids And Liquids Coexist At Their Melting Point.
From www.animalia-life.club
Examples Of Solids Liquids And Gases Why Do Solids And Liquids Coexist At Their Melting Point More heat then will convert the solid into a liquid with no temperature change. Assertion :the melting point is defined for solids when it converts from solid state to. A phase transition like this, in which a solid compound changes into a liquid and a different solid, is called incongruent or. As heat is applied to a solid, its temperature. Why Do Solids And Liquids Coexist At Their Melting Point.
From philschatz.com
Phase Diagrams · Chemistry Why Do Solids And Liquids Coexist At Their Melting Point The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. Hence, solids and liquids co. It is unique to a substance and is dependent on the pressure acting on the body. Greater the pressure, the lower the melting point. At the melting point, the solid and liquid phase exist in: As heat is. Why Do Solids And Liquids Coexist At Their Melting Point.
From www.coursehero.com
Solid to Gas Phase Transition Introduction to Chemistry Course Hero Why Do Solids And Liquids Coexist At Their Melting Point More heat then will convert the solid into a liquid with no temperature change. As heat is applied to a solid, its temperature will increase until the melting point is reached. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). Greater the pressure, the. Why Do Solids And Liquids Coexist At Their Melting Point.
From exoufpnzr.blob.core.windows.net
Liquid To Solid Called at Allan Quinn blog Why Do Solids And Liquids Coexist At Their Melting Point Hence, solids and liquids co. At the melting point, the solid and liquid phase exist in: The temperature at which solid and liquid states coexist together is called the melting point. It is unique to a substance and is dependent on the pressure acting on the body. A phase transition like this, in which a solid compound changes into a. Why Do Solids And Liquids Coexist At Their Melting Point.
From circuitlibbottega.z21.web.core.windows.net
How To Determine Melting Point Chemistry Why Do Solids And Liquids Coexist At Their Melting Point Hence, solids and liquids co. At the melting point, the solid and liquid phase exist in: The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. The temperature at which solid and liquid states coexist together is called the melting point. A phase transition like this, in which a solid compound changes into. Why Do Solids And Liquids Coexist At Their Melting Point.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Why Do Solids And Liquids Coexist At Their Melting Point Hence, solids and liquids co. A phase transition like this, in which a solid compound changes into a liquid and a different solid, is called incongruent or. It is unique to a substance and is dependent on the pressure acting on the body. Greater the pressure, the lower the melting point. As heat is applied to a solid, its temperature. Why Do Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
Liquids and Solids Chapter ppt download Why Do Solids And Liquids Coexist At Their Melting Point At the melting point, the solid and liquid phase exist in: The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. As heat is applied to a solid, its temperature will increase until the melting point is reached. Assertion :the melting point is defined for solids when it converts from solid state to.. Why Do Solids And Liquids Coexist At Their Melting Point.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID Why Do Solids And Liquids Coexist At Their Melting Point It is unique to a substance and is dependent on the pressure acting on the body. At the melting point, the solid and liquid phase exist in: Assertion :the melting point is defined for solids when it converts from solid state to. More heat then will convert the solid into a liquid with no temperature change. The temperature at which. Why Do Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
States and Changes of Matter ppt download Why Do Solids And Liquids Coexist At Their Melting Point The temperature at which solid and liquid states coexist together is called the melting point. Greater the pressure, the lower the melting point. More heat then will convert the solid into a liquid with no temperature change. As heat is applied to a solid, its temperature will increase until the melting point is reached. At the melting point, the solid. Why Do Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
Phase Changes. ppt download Why Do Solids And Liquids Coexist At Their Melting Point At the melting point, the solid and liquid phase exist in: The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. As heat is applied to a solid, its temperature will increase until the melting point is reached. Assertion :the melting point is defined for solids when it converts from solid state to.. Why Do Solids And Liquids Coexist At Their Melting Point.
From loeaanczy.blob.core.windows.net
Chlorine Boiling Point And Melting Point at Jane Gibbs blog Why Do Solids And Liquids Coexist At Their Melting Point It is unique to a substance and is dependent on the pressure acting on the body. A phase transition like this, in which a solid compound changes into a liquid and a different solid, is called incongruent or. As heat is applied to a solid, its temperature will increase until the melting point is reached. Melting point, temperature at which. Why Do Solids And Liquids Coexist At Their Melting Point.
From general.chemistrysteps.com
States of Matter Solid, Liquid, Gas, and Plasma Chemistry Steps Why Do Solids And Liquids Coexist At Their Melting Point A phase transition like this, in which a solid compound changes into a liquid and a different solid, is called incongruent or. As heat is applied to a solid, its temperature will increase until the melting point is reached. Hence, solids and liquids co. More heat then will convert the solid into a liquid with no temperature change. The temperature. Why Do Solids And Liquids Coexist At Their Melting Point.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Why Do Solids And Liquids Coexist At Their Melting Point As heat is applied to a solid, its temperature will increase until the melting point is reached. Melting point, temperature at which the solid and liquid forms of a pure substance can exist in equilibrium. More heat then will convert the solid into a liquid with no temperature change. The temperature at which solid and liquid states coexist together is. Why Do Solids And Liquids Coexist At Their Melting Point.
From www.pinterest.com
Melting Point Easy Science Chemistry education, Learn physics Why Do Solids And Liquids Coexist At Their Melting Point More heat then will convert the solid into a liquid with no temperature change. The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. Hence, solids and liquids co. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy. Why Do Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
Liquids & Vapor Pressure ppt download Why Do Solids And Liquids Coexist At Their Melting Point The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). Greater the pressure, the lower the melting point. At the melting point, the solid and liquid phase exist in: A phase transition like this, in which a solid compound changes into a liquid and a. Why Do Solids And Liquids Coexist At Their Melting Point.
From www.teachoo.com
Effect of Temperature to Change State of Matter Teachoo Science Why Do Solids And Liquids Coexist At Their Melting Point At the melting point, the solid and liquid phase exist in: The temperature at which solid and liquid states coexist together is called the melting point. Hence, solids and liquids co. The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. A phase transition like this, in which a solid compound changes into. Why Do Solids And Liquids Coexist At Their Melting Point.
From www.snexplores.org
Explainer What are the different states of matter? Why Do Solids And Liquids Coexist At Their Melting Point Assertion :the melting point is defined for solids when it converts from solid state to. More heat then will convert the solid into a liquid with no temperature change. As heat is applied to a solid, its temperature will increase until the melting point is reached. Melting point, temperature at which the solid and liquid forms of a pure substance. Why Do Solids And Liquids Coexist At Their Melting Point.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID Why Do Solids And Liquids Coexist At Their Melting Point The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. The melting point is the temperature where the solid and liquid phases are in equilibrium with each other, and the change in free energy (δgo). The temperature at which solid and liquid states coexist together is called the melting point. Hence, solids and. Why Do Solids And Liquids Coexist At Their Melting Point.
From slideplayer.com
Intermolecular Forces and ppt download Why Do Solids And Liquids Coexist At Their Melting Point Assertion :the melting point is defined for solids when it converts from solid state to. The pressure controls the rate of melting by influencing the equilibrium of the solid and liquid states. More heat then will convert the solid into a liquid with no temperature change. The temperature at which solid and liquid states coexist together is called the melting. Why Do Solids And Liquids Coexist At Their Melting Point.