Does Magnesium Form A Positive Ion . When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and. Magnesium atoms contain 12 protons in their nucleus. Metals form positive ions (cations). The ions are positive, because they. Metal atoms lose electrons from their outer shell when they form ions: Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. To get a +2 charge, the ion had to lose two electrons. Ionic charges of all elements (list). A magnesium atom must lose two electrons to have the same number electrons as an atom of.
from fineartamerica.com
A magnesium atom must lose two electrons to have the same number electrons as an atom of. Metal atoms lose electrons from their outer shell when they form ions: Write the symbol for each ion and. Metals form positive ions (cations). When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Magnesium and nitrogen react to form an ionic compound. The ions are positive, because they. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium atoms contain 12 protons in their nucleus. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the.
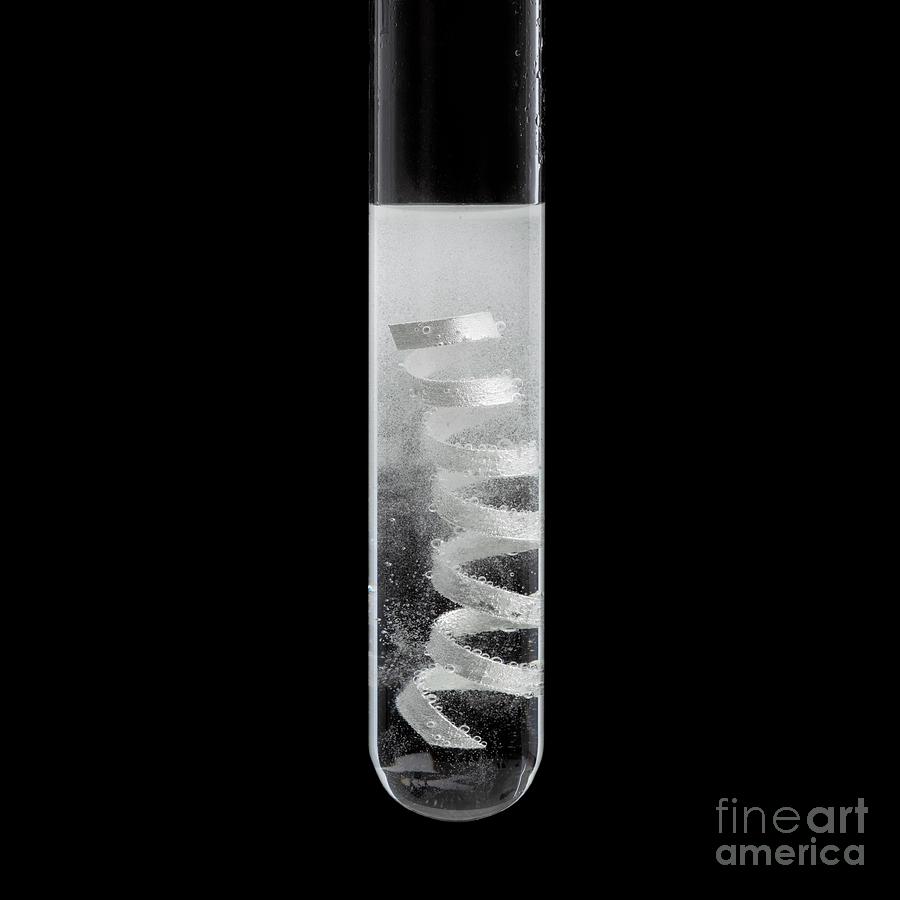
Magnesiumacid Reaction Photograph by Science Photo Library Fine Art
Does Magnesium Form A Positive Ion The ions are positive, because they. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. Metal atoms lose electrons from their outer shell when they form ions: Predict which forms an anion, which forms a cation, and the charges of each ion. A magnesium atom must lose two electrons to have the same number electrons as an atom of. Ionic charges of all elements (list). Magnesium atoms contain 12 protons in their nucleus. The ions are positive, because they. To get a +2 charge, the ion had to lose two electrons. Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and. Metals form positive ions (cations).
From katiecouric.com
Does Magnesium Help You Sleep? Best Magnesium for Sleep Does Magnesium Form A Positive Ion Metal atoms lose electrons from their outer shell when they form ions: Write the symbol for each ion and. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. Metals form positive ions (cations). Ionic charges of all elements (list). Magnesium atoms contain. Does Magnesium Form A Positive Ion.
From www.pinterest.co.uk
“Top 10 sources of magnesium supplements” Foods high in magnesium Does Magnesium Form A Positive Ion Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. A magnesium atom must lose two electrons to have the same. Does Magnesium Form A Positive Ion.
From trueufiles204.weebly.com
Magnesium Valence Electrons trueufiles Does Magnesium Form A Positive Ion Metals form positive ions (cations). Write the symbol for each ion and. The ions are positive, because they. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium (2,8,2) forms a 2+. Does Magnesium Form A Positive Ion.
From www.healthydirections.com
Benefits of Magnesium No One Told You About Healthy Directions Does Magnesium Form A Positive Ion The ions are positive, because they. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Write the symbol for each ion and. To get a +2 charge, the ion had to lose two electrons. Predict which forms an anion, which forms a cation, and the charges of. Does Magnesium Form A Positive Ion.
From www.dailybody.net
What Does Magnesium And Nitrogen Make? (Detailed Guide) Does Magnesium Form A Positive Ion Ionic charges of all elements (list). Write the symbol for each ion and. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. To get a +2 charge, the ion had to lose two electrons. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra. Does Magnesium Form A Positive Ion.
From brookswlcd651.weebly.com
7 Best Magnesium Supplements 2021, According To Health The best Does Magnesium Form A Positive Ion Magnesium and nitrogen react to form an ionic compound. A magnesium atom must lose two electrons to have the same number electrons as an atom of. Write the symbol for each ion and. The ions are positive, because they. Magnesium atoms contain 12 protons in their nucleus. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra. Does Magnesium Form A Positive Ion.
From www.youtube.com
What does Magnesium Ore Look Like? YouTube Does Magnesium Form A Positive Ion Magnesium and nitrogen react to form an ionic compound. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Magnesium atoms contain 12 protons in their nucleus. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is. Does Magnesium Form A Positive Ion.
From www.pinterest.com
Magnesium Supplements Explained Which One is Best for Your Type of Does Magnesium Form A Positive Ion To get a +2 charge, the ion had to lose two electrons. Write the symbol for each ion and. Ionic charges of all elements (list). Magnesium atoms contain 12 protons in their nucleus. The ions are positive, because they. A magnesium atom must lose two electrons to have the same number electrons as an atom of. When atoms gain electron/s,. Does Magnesium Form A Positive Ion.
From fineartamerica.com
Magnesiumacid Reaction Photograph by Science Photo Library Fine Art Does Magnesium Form A Positive Ion Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. A magnesium atom must lose two electrons to have the same number electrons as an atom of. Metal atoms lose electrons from their outer shell when they form ions: Metals form positive ions. Does Magnesium Form A Positive Ion.
From www.13clouds.com
Computer the vital the identify betw economic examples previously by Does Magnesium Form A Positive Ion Predict which forms an anion, which forms a cation, and the charges of each ion. A magnesium atom must lose two electrons to have the same number electrons as an atom of. The ions are positive, because they. Metal atoms lose electrons from their outer shell when they form ions: Metals form positive ions (cations). Ionic charges of all elements. Does Magnesium Form A Positive Ion.
From www.pinterest.com.au
Benefits of Magnesium For Healthier Hormones And Periods Healthy Does Magnesium Form A Positive Ion Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium and nitrogen react to form an ionic compound. Metal atoms lose electrons from their outer shell when they form ions: A magnesium atom must lose two electrons to have the same number electrons as an atom of. The ions are positive, because they. To. Does Magnesium Form A Positive Ion.
From www.msn.com
Health Benefits of Magnesium Does Magnesium Form A Positive Ion Metal atoms lose electrons from their outer shell when they form ions: Metals form positive ions (cations). Predict which forms an anion, which forms a cation, and the charges of each ion. A magnesium atom must lose two electrons to have the same number electrons as an atom of. Write the symbol for each ion and. Ionic charges of all. Does Magnesium Form A Positive Ion.
From www.curamedicine.com.au
Does Magnesium Cause Digestive Issues? Does Magnesium Form A Positive Ion Write the symbol for each ion and. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Ionic charges of all elements (list). Magnesium atoms contain 12 protons in their nucleus. A magnesium atom must lose two electrons to have the same number electrons as an atom of.. Does Magnesium Form A Positive Ion.
From symbiosis.co.nz
Magnesium Symbiosis Agriculture Does Magnesium Form A Positive Ion To get a +2 charge, the ion had to lose two electrons. Metals form positive ions (cations). The ions are positive, because they. Write the symbol for each ion and. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium atoms contain 12 protons in their nucleus. When atoms gain electron/s, the negatively charged. Does Magnesium Form A Positive Ion.
From www.ccdc.cam.ac.uk
Magnesium CCDC Does Magnesium Form A Positive Ion Magnesium atoms contain 12 protons in their nucleus. Metals form positive ions (cations). Metal atoms lose electrons from their outer shell when they form ions: Magnesium and nitrogen react to form an ionic compound. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. A magnesium atom must. Does Magnesium Form A Positive Ion.
From www.youtube.com
Why does Magnesium react with Water? YouTube Does Magnesium Form A Positive Ion Magnesium and nitrogen react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of each ion. Metal atoms lose electrons from their outer shell when they form ions: Magnesium atoms contain 12 protons in their nucleus. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s,. Does Magnesium Form A Positive Ion.
From www.pinterest.com
What will Magnesium help me with? How long will it take? Tune in to Does Magnesium Form A Positive Ion Metals form positive ions (cations). To get a +2 charge, the ion had to lose two electrons. The ions are positive, because they. Write the symbol for each ion and. Metal atoms lose electrons from their outer shell when they form ions: When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively. Does Magnesium Form A Positive Ion.
From kristiansabols.github.io
Ķīmija Does Magnesium Form A Positive Ion Predict which forms an anion, which forms a cation, and the charges of each ion. Ionic charges of all elements (list). When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. To get a +2 charge, the ion had to lose two electrons. Metals form positive ions (cations).. Does Magnesium Form A Positive Ion.
From thefitnessmanual.com
How Does Magnesium Chloride Form TheFitnessManual Does Magnesium Form A Positive Ion Metal atoms lose electrons from their outer shell when they form ions: Metals form positive ions (cations). Predict which forms an anion, which forms a cation, and the charges of each ion. The ions are positive, because they. A magnesium atom must lose two electrons to have the same number electrons as an atom of. When atoms gain electron/s, the. Does Magnesium Form A Positive Ion.
From wholisticmatters.com
Magnesium The Nutrient WholisticMatters Does Magnesium Form A Positive Ion Metal atoms lose electrons from their outer shell when they form ions: Magnesium and nitrogen react to form an ionic compound. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. When atoms gain electron/s, the negatively charged ion is formed, and when. Does Magnesium Form A Positive Ion.
From www.neuroneeds.com
MAGNESIUM Neuroneeds Does Magnesium Form A Positive Ion A magnesium atom must lose two electrons to have the same number electrons as an atom of. Magnesium atoms contain 12 protons in their nucleus. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. Write the symbol for each ion and. To. Does Magnesium Form A Positive Ion.
From storage.googleapis.com
Does magnesium improve mood? Does Magnesium Form A Positive Ion Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. Predict which forms an anion, which forms a cation, and the charges of each ion. The ions are positive, because they. Magnesium atoms contain 12 protons in their nucleus. A magnesium atom must. Does Magnesium Form A Positive Ion.
From www.patchmd.com
How Long Does Magnesium Stay in Your Body? A Complete Guide to Does Magnesium Form A Positive Ion Magnesium and nitrogen react to form an ionic compound. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. The ions are positive, because they. Magnesium atoms contain 12 protons in their nucleus. Metal atoms lose electrons from their outer shell when they. Does Magnesium Form A Positive Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does Magnesium Form A Positive Ion Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. Predict which forms an anion, which forms a cation, and the charges of each ion. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively. Does Magnesium Form A Positive Ion.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Does Magnesium Form A Positive Ion Ionic charges of all elements (list). When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. The ions are positive, because they. Predict which forms an anion, which forms a cation, and the charges of each ion. To get a +2 charge, the ion had to lose two. Does Magnesium Form A Positive Ion.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Does Magnesium Form A Positive Ion Write the symbol for each ion and. Ionic charges of all elements (list). The ions are positive, because they. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Magnesium atoms contain 12 protons in their nucleus. Metal atoms lose electrons from their outer shell when they form. Does Magnesium Form A Positive Ion.
From www.mdpi.com
Nutrients Free FullText Magnesium Deficiency and Cardiometabolic Does Magnesium Form A Positive Ion Metal atoms lose electrons from their outer shell when they form ions: Magnesium and nitrogen react to form an ionic compound. Write the symbol for each ion and. Ionic charges of all elements (list). Metals form positive ions (cations). Magnesium atoms contain 12 protons in their nucleus. To get a +2 charge, the ion had to lose two electrons. The. Does Magnesium Form A Positive Ion.
From ictchemskool.blogspot.com
SkooL of CheM Does Magnesium Form A Positive Ion To get a +2 charge, the ion had to lose two electrons. Predict which forms an anion, which forms a cation, and the charges of each ion. The ions are positive, because they. When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Metals form positive ions (cations).. Does Magnesium Form A Positive Ion.
From essanews.com
Magnesium deficiency 4 signs you may be ignoring and how to prevent it Does Magnesium Form A Positive Ion To get a +2 charge, the ion had to lose two electrons. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. The ions are positive, because they. Write the symbol for each ion and. Metals form positive ions (cations). Magnesium atoms contain. Does Magnesium Form A Positive Ion.
From focussupplements.co.uk
Demystifying The Many Forms Of Magnesium Focus Supplements Does Magnesium Form A Positive Ion Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium atoms contain 12 protons in their nucleus. Metals form positive ions (cations). To get a +2 charge, the ion had to lose two electrons. The ions are positive, because they. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy. Does Magnesium Form A Positive Ion.
From www.pinterest.com
Top 10 Surprising Magnesium Benefits Magnesium benefits, Lemon Does Magnesium Form A Positive Ion When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Write the symbol for each ion and. Metals form positive ions (cations). A magnesium atom must lose two electrons to have the same number electrons as an atom of. Magnesium and nitrogen react to form an ionic compound.. Does Magnesium Form A Positive Ion.
From cabinet.matttroy.net
Properties Of Magnesium On The Periodic Table Matttroy Does Magnesium Form A Positive Ion Ionic charges of all elements (list). When atoms gain electron/s, the negatively charged ion is formed, and when the atoms lose electron/s, the positively charged ion is formed. Magnesium atoms contain 12 protons in their nucleus. Write the symbol for each ion and. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove. Does Magnesium Form A Positive Ion.
From www.dailybody.net
Is Magnesium Positive Or Negative? (Read This First!) Does Magnesium Form A Positive Ion Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. The ions are positive, because they. Magnesium atoms contain 12 protons in their nucleus. Metals form positive ions (cations). Predict which forms an anion, which forms a cation, and the charges of each. Does Magnesium Form A Positive Ion.
From cloudmineinc.com
How Long Does Magnesium Stay In Your Body? Does Magnesium Form A Positive Ion To get a +2 charge, the ion had to lose two electrons. Predict which forms an anion, which forms a cation, and the charges of each ion. Magnesium atoms contain 12 protons in their nucleus. The ions are positive, because they. Ionic charges of all elements (list). Magnesium and nitrogen react to form an ionic compound. Magnesium (2,8,2) forms a. Does Magnesium Form A Positive Ion.
From design.udlvirtual.edu.pe
What Does It Mean When An Ion Has A Positive Charge Design Talk Does Magnesium Form A Positive Ion To get a +2 charge, the ion had to lose two electrons. Magnesium (2,8,2) forms a 2+ rather than a 1+ ion because the extra energy needed to remove the second electron is more than made up by the. Ionic charges of all elements (list). Metals form positive ions (cations). Write the symbol for each ion and. Predict which forms. Does Magnesium Form A Positive Ion.