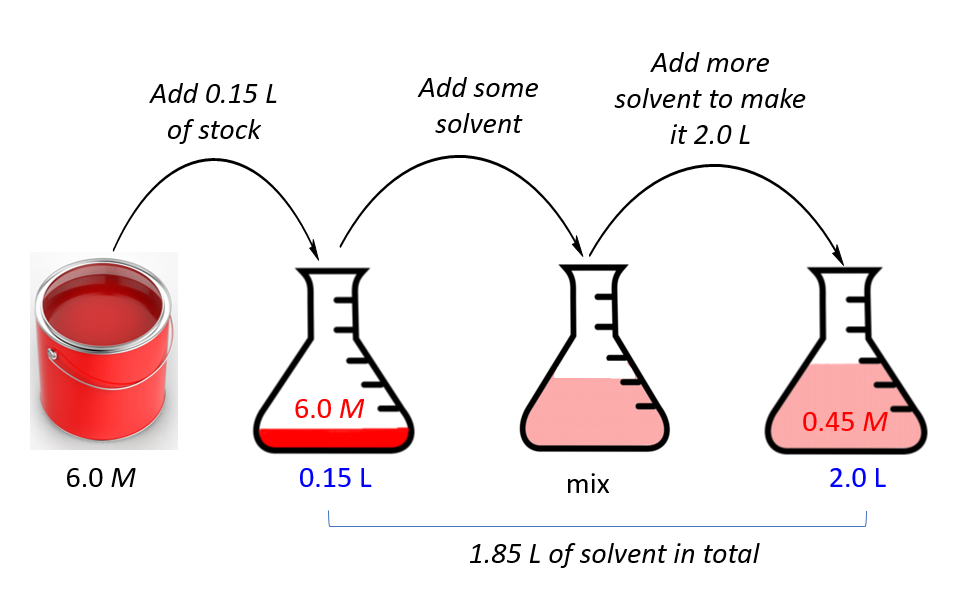
Dilution Practice Problems Chemistry . Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute. There’s a bottle of 0.750 m nacl on a shelf. What was the beginning volume of the 20% solution?, you dilute 50 ml of an 8% solution to 500 ml. These dilution example problems show how to perform the calculations needed to make a diluted solution. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0 ml of the diluted solution contains 5.09 g of kcl. What is the percentage strength of the. The key idea behind a dilution is. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. The number of moles always stays the same. How much of it do you need to prepare 50 ml of a. Dilution m 1v 1=m 2v 2 1.
from general.chemistrysteps.com
Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0 ml of the diluted solution contains 5.09 g of kcl. The key idea behind a dilution is. To dilute a solution means to add more solvent without the addition of more solute. The number of moles always stays the same. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. How much of it do you need to prepare 50 ml of a. Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. Of course, the resulting solution is thoroughly mixed so as to. What is the percentage strength of the.
Dilution of a Stock Solution and Calculations Based Morality
Dilution Practice Problems Chemistry Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to add more solvent without the addition of more solute. What is the percentage strength of the. These dilution example problems show how to perform the calculations needed to make a diluted solution. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. The key idea behind a dilution is. What was the beginning volume of the 20% solution?, you dilute 50 ml of an 8% solution to 500 ml. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0 ml of the diluted solution contains 5.09 g of kcl. There’s a bottle of 0.750 m nacl on a shelf. Dilution m 1v 1=m 2v 2 1. How much of it do you need to prepare 50 ml of a. Of course, the resulting solution is thoroughly mixed so as to. The number of moles always stays the same.
From www.chemistryworksheet.com
Dilution Problems Chemistry Worksheet With Answers Dilution Practice Problems Chemistry How much of it do you need to prepare 50 ml of a. The number of moles always stays the same. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Of course, the resulting solution is thoroughly mixed so as to. To dilute a solution means to. Dilution Practice Problems Chemistry.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Dilution Practice Problems Chemistry The key idea behind a dilution is. To dilute a solution means to add more solvent without the addition of more solute. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. The number of moles always stays the same. There’s a bottle of 0.750 m nacl on. Dilution Practice Problems Chemistry.
From www.worksheeto.com
7 Molarity Worksheet With Answers / Dilution Practice Problems Chemistry To dilute a solution means to add more solvent without the addition of more solute. There’s a bottle of 0.750 m nacl on a shelf. The number of moles always stays the same. Dilution m 1v 1=m 2v 2 1. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80. Dilution Practice Problems Chemistry.
From lessonmagicnunez.z21.web.core.windows.net
Molarity And Dilution Worksheets Dilution Practice Problems Chemistry The key idea behind a dilution is. Dilution m 1v 1=m 2v 2 1. The number of moles always stays the same. What is the percentage strength of the. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Of course, the resulting solution is thoroughly mixed so. Dilution Practice Problems Chemistry.
From www.chegg.com
Solved Dilution Practice ProblemsSolve the following Dilution Practice Problems Chemistry Of course, the resulting solution is thoroughly mixed so as to. What is the percentage strength of the. To dilute a solution means to add more solvent without the addition of more solute. The number of moles always stays the same. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to. Dilution Practice Problems Chemistry.
From www.docsity.com
Making Dilutions Worksheet Answers Key Docsity Dilution Practice Problems Chemistry Dilution m 1v 1=m 2v 2 1. There’s a bottle of 0.750 m nacl on a shelf. Of course, the resulting solution is thoroughly mixed so as to. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. What was the beginning volume of the 20% solution?, you dilute. Dilution Practice Problems Chemistry.
From www.youtube.com
Solving Dilution Problems in Solution Chemistry CLEAR & SIMPLE YouTube Dilution Practice Problems Chemistry What is the percentage strength of the. The number of moles always stays the same. Dilution m 1v 1=m 2v 2 1. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. There’s a bottle of 0.750 m nacl on a shelf. These dilution example problems show how. Dilution Practice Problems Chemistry.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Practice Problems Chemistry If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0 ml of the diluted solution contains 5.09 g of kcl. Of course, the resulting solution is thoroughly mixed so as to.. Dilution Practice Problems Chemistry.
From www.studocu.com
Dilutions Worksheet Dilution problems CHEM 1010 Studocu Dilution Practice Problems Chemistry Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0 ml of the diluted solution contains 5.09 g of kcl. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Dilution m 1v 1=m 2v 2 1. What was the beginning. Dilution Practice Problems Chemistry.
From www.youtube.com
Molarity and Dilution Practice Problems YouTube Dilution Practice Problems Chemistry What was the beginning volume of the 20% solution?, you dilute 50 ml of an 8% solution to 500 ml. To dilute a solution means to add more solvent without the addition of more solute. Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0 ml of the diluted solution contains 5.09 g. Dilution Practice Problems Chemistry.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Practice Problems Chemistry Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. To dilute a solution means to add more solvent without the addition of more solute. These dilution example problems show how to perform the calculations needed to make a diluted solution. What was the beginning volume of the 20%. Dilution Practice Problems Chemistry.
From joiuugyhn.blob.core.windows.net
Dilution Calculator Concentration at Bernard Butler blog Dilution Practice Problems Chemistry Of course, the resulting solution is thoroughly mixed so as to. These dilution example problems show how to perform the calculations needed to make a diluted solution. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. To dilute a solution means to add more solvent without the addition. Dilution Practice Problems Chemistry.
From www.youtube.com
Dilution Practice Problems 4 & 5 YouTube Dilution Practice Problems Chemistry How much of it do you need to prepare 50 ml of a. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. What was the beginning volume of the 20% solution?, you dilute 50 ml of an 8% solution to 500 ml. There’s a bottle of 0.750 m. Dilution Practice Problems Chemistry.
From www.studocu.com
1. Serial Dilution Calculations Dilution Plating Questions Question Dilution Practice Problems Chemistry These dilution example problems show how to perform the calculations needed to make a diluted solution. What is the percentage strength of the. There’s a bottle of 0.750 m nacl on a shelf. To dilute a solution means to add more solvent without the addition of more solute. How much of it do you need to prepare 50 ml of. Dilution Practice Problems Chemistry.
From www.studocu.com
Molarity & Dilutions Practice Problems Answers Molarity & Dilution Dilution Practice Problems Chemistry These dilution example problems show how to perform the calculations needed to make a diluted solution. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf.. Dilution Practice Problems Chemistry.
From kidsworksheetfun.com
Concentrations And Dilutions Worksheet Answers Kidsworksheetfun Dilution Practice Problems Chemistry The number of moles always stays the same. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0 ml of the diluted solution contains 5.09 g of kcl. What is the. Dilution Practice Problems Chemistry.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Dilution Practice Problems Chemistry The key idea behind a dilution is. Dilution m 1v 1=m 2v 2 1. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. How much of it do you need to prepare 50 ml of a. What was the beginning volume of the 20% solution?, you dilute. Dilution Practice Problems Chemistry.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution Practice Problems Chemistry The number of moles always stays the same. The key idea behind a dilution is. To dilute a solution means to add more solvent without the addition of more solute. What is the percentage strength of the. Dilution m 1v 1=m 2v 2 1. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine. Dilution Practice Problems Chemistry.
From exopkbipv.blob.core.windows.net
Dilution Equation Example Chemistry at Wanda King blog Dilution Practice Problems Chemistry The key idea behind a dilution is. To dilute a solution means to add more solvent without the addition of more solute. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. These dilution example problems show how to perform the calculations needed to make a diluted solution. How. Dilution Practice Problems Chemistry.
From materialdietrich.z19.web.core.windows.net
Molarity Worksheet Answer Key Chemistry Dilution Practice Problems Chemistry Of course, the resulting solution is thoroughly mixed so as to. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. What was the beginning volume of the 20% solution?, you dilute 50 ml of an 8% solution to 500 ml. If you dilute 175 ml of a 1.6. Dilution Practice Problems Chemistry.
From joivmtjnj.blob.core.windows.net
How To Make Dilution From Stock Solution at Dorothy Grenier blog Dilution Practice Problems Chemistry To dilute a solution means to add more solvent without the addition of more solute. What was the beginning volume of the 20% solution?, you dilute 50 ml of an 8% solution to 500 ml. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Of course, the resulting. Dilution Practice Problems Chemistry.
From printablemediaells.z21.web.core.windows.net
Dilution Worksheet Chemistry Dilution Practice Problems Chemistry Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Of course, the resulting solution is thoroughly mixed so as to. The number of moles always stays the same. How much of it do you need to prepare 50 ml of a. What was the beginning volume of the. Dilution Practice Problems Chemistry.
From www.docsity.com
Molarity and Dilutions Practice Worksheet Key Docsity Dilution Practice Problems Chemistry Of course, the resulting solution is thoroughly mixed so as to. Dilution m 1v 1=m 2v 2 1. These dilution example problems show how to perform the calculations needed to make a diluted solution. The number of moles always stays the same. What is the percentage strength of the. The key idea behind a dilution is. How much of it. Dilution Practice Problems Chemistry.
From www.youtube.com
Molarity Dilution Practice Problem 2 YouTube Dilution Practice Problems Chemistry There’s a bottle of 0.750 m nacl on a shelf. The number of moles always stays the same. Of course, the resulting solution is thoroughly mixed so as to. These dilution example problems show how to perform the calculations needed to make a diluted solution. What was the beginning volume of the 20% solution?, you dilute 50 ml of an. Dilution Practice Problems Chemistry.
From www.youtube.com
Dilution Practice Problems & Example Problems YouTube Dilution Practice Problems Chemistry Dilution m 1v 1=m 2v 2 1. These dilution example problems show how to perform the calculations needed to make a diluted solution. What was the beginning volume of the 20% solution?, you dilute 50 ml of an 8% solution to 500 ml. Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0. Dilution Practice Problems Chemistry.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Practice Problems Chemistry Dilution m 1v 1=m 2v 2 1. How much of it do you need to prepare 50 ml of a. The key idea behind a dilution is. Of course, the resulting solution is thoroughly mixed so as to. Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Determine. Dilution Practice Problems Chemistry.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Practice Problems Chemistry If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. The key idea behind a dilution is. Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0 ml of the diluted solution contains 5.09 g of kcl. Dilution m 1v 1=m. Dilution Practice Problems Chemistry.
From www.chegg.com
Solved Serial dilution is a common technique used in Dilution Practice Problems Chemistry What is the percentage strength of the. The key idea behind a dilution is. Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0 ml of the diluted solution contains 5.09 g of kcl. Of course, the resulting solution is thoroughly mixed so as to. If you dilute 175 ml of a 1.6. Dilution Practice Problems Chemistry.
From studylib.net
Key DilutionsWorksheet Dilution Practice Problems Chemistry Explain what changes and what stays the same when 1.00 l of a solution of nacl is diluted to 1.80 l. Dilution m 1v 1=m 2v 2 1. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. What is the percentage strength of the. Of course, the. Dilution Practice Problems Chemistry.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Practice Problems Chemistry What is the percentage strength of the. Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0 ml of the diluted solution contains 5.09 g of kcl. Of course, the resulting solution is thoroughly mixed so as to. How much of it do you need to prepare 50 ml of a. Explain what. Dilution Practice Problems Chemistry.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution Practice Problems Chemistry Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution so that 50.0 ml of the diluted solution contains 5.09 g of kcl. What is the percentage strength of the. The number of moles always stays the same. How much of it do you need to prepare 50 ml of a. Explain what changes and what. Dilution Practice Problems Chemistry.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Practice Problems Chemistry The number of moles always stays the same. What is the percentage strength of the. To dilute a solution means to add more solvent without the addition of more solute. There’s a bottle of 0.750 m nacl on a shelf. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of. Dilution Practice Problems Chemistry.
From www.chegg.com
Solved Lab 5 Practice Dilution Problems Q1 You have a Dilution Practice Problems Chemistry There’s a bottle of 0.750 m nacl on a shelf. Dilution m 1v 1=m 2v 2 1. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. What is the percentage strength of the. Determine the volume you should dilute 78.0 ml of a 3.50 m kcl solution. Dilution Practice Problems Chemistry.
From exoxgkatn.blob.core.windows.net
Dilution Problems With Answers at Michael Keller blog Dilution Practice Problems Chemistry There’s a bottle of 0.750 m nacl on a shelf. These dilution example problems show how to perform the calculations needed to make a diluted solution. How much of it do you need to prepare 50 ml of a. What was the beginning volume of the 20% solution?, you dilute 50 ml of an 8% solution to 500 ml. The. Dilution Practice Problems Chemistry.
From pdfprof.com
concentration of solutions practice problems Dilution Practice Problems Chemistry How much of it do you need to prepare 50 ml of a. There’s a bottle of 0.750 m nacl on a shelf. If you dilute 175 ml of a 1.6 m solution of licl to 1.0 l, determine the new concentration of the solution. The key idea behind a dilution is. Dilution m 1v 1=m 2v 2 1. To. Dilution Practice Problems Chemistry.