High Acid Value . It is defined as the weight of koh in mg needed to neutralize the organic acids present in 1g of fat and it is a measure of the free fatty acids (ffa) present in the fat or oil. the acidity or alkalinity of foods are measured by their ph, and it is measured on a scale of 0 to 14. the acid value (av) is a common parameter in the specification of fats and oils. definitions of ph, poh, and the ph scale. the acid number is the quantity of base, expressed in milligrams of potassium hydroxide, which is required to neutralize. acid value (av), also known as acid number, neutralization number, or acidity, is a quantity used in chemistry to express how acidic a certain chemical compound is. Acid value is an important indicator of vegetable oil quality. thus, the methoxide anion is the most stable (lowest energy, least basic) of the three conjugate bases, and the ethyl carbanion anion is the least stable (highest. the acid value and the free fatty acid content are important parameters used for the characterization and the quality. the acid value is defined as mg of naoh required to neutralize the free fatty acids in 1 g of vegetable oil. acid value (av) is employed to evaluate free fatty acid content and is a significant factor in determining the refining degree and. The glycerides are also hydrolysed with water in the. It’s the amount of base typically potassium hydroxide (koh) expressed in milligrams that is necessary to neutralize the acidic components in 1 gram of a sample. Acid value is expressed as the amount of potassium. Uric acid is a waste product formed.
from www.preclaboratories.com
the acid value (av) is a common parameter in the specification of fats and oils. high acid food and drink. the acid number is the quantity of base, expressed in milligrams of potassium hydroxide, which is required to neutralize. Acid value is an important indicator of vegetable oil quality. It is the quantity of. in chemistry, acid value (av, acid number, neutralization number or acidity) is a number used to quantify the acidity of a given. To know the relationship between acid or base strength and the magnitude of \ (k_a\), \. It is defined as the weight of koh in mg needed to neutralize the organic acids present in 1g of fat and it is a measure of the free fatty acids (ffa) present in the fat or oil. the ph scale shows how acidic or basic a chemical is in aqueous solution (mixed with water). the acid value is a measure of the extent to which the glycerides in the oil have been hydrolysed by lipase action.
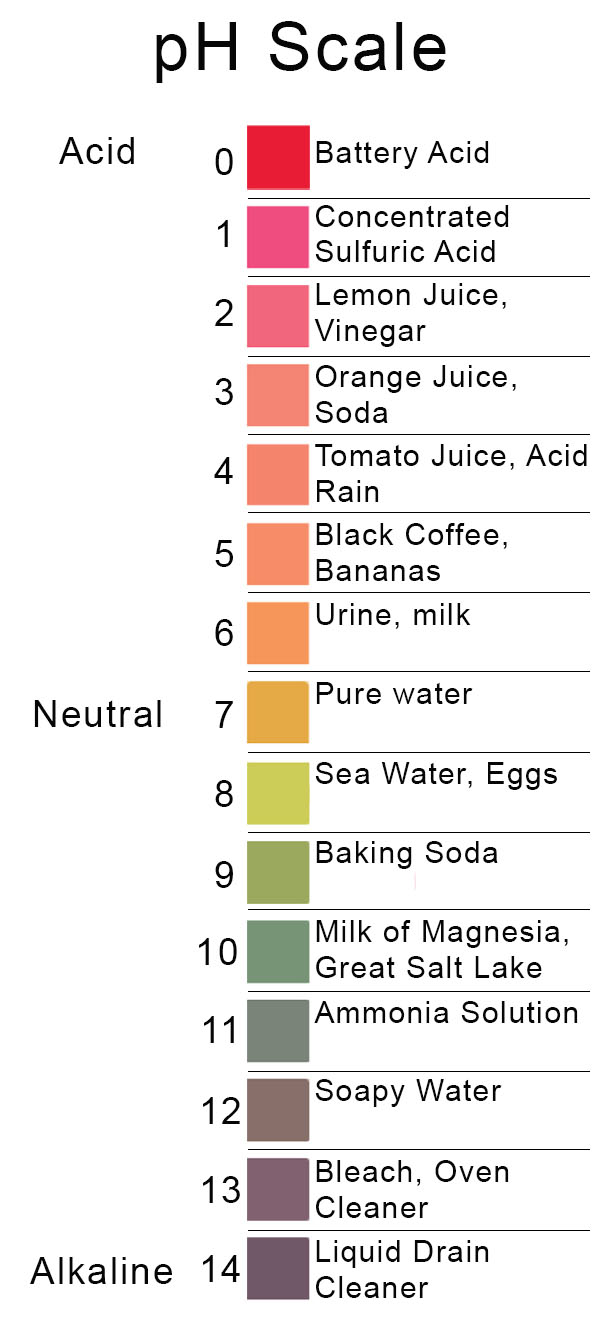
Back to Basics Acids, Bases & the pH Scale Precision Laboratories
High Acid Value the acidity or alkalinity of foods are measured by their ph, and it is measured on a scale of 0 to 14. To know the relationship between acid or base strength and the magnitude of \ (k_a\), \. It is the quantity of. It is expressed as the amount of potassium hydroxide (koh) required to neutralize the free fatty acids present in one gram of the substance. the acid value is defined as mg of naoh required to neutralize the free fatty acids in 1 g of vegetable oil. the acid value is a measure of the extent to which the glycerides in the oil have been hydrolysed by lipase action. Calculating the ph of a strong acid or base solution. It is defined as the weight of koh in mg needed to neutralize the organic acids present in 1g of fat and it is a measure of the free fatty acids (ffa) present in the fat or oil. the ph scale shows how acidic or basic a chemical is in aqueous solution (mixed with water). The glycerides are also hydrolysed with water in the. high tan values indicate a higher acid content, which can lead to accelerated equipment degradation and. acid value (av) is employed to evaluate free fatty acid content and is a significant factor in determining the refining degree and. Uric acid is a waste product formed. But the ph of food before. the amount of free fatty acids that are present in a substance typically a fat or oil is measured by its acid value and called as acid value in fats and oils. It’s the amount of base typically potassium hydroxide (koh) expressed in milligrams that is necessary to neutralize the acidic components in 1 gram of a sample.
From chemistry.stackexchange.com
everyday chemistry Sugar solutions have a neutral pH in themselves High Acid Value the acid number is used to quantify the amount of acid present, for example, in a sample of biodiesel. the ph scale shows how acidic or basic a chemical is in aqueous solution (mixed with water). Acid value is an important indicator of vegetable oil quality. To know the relationship between acid or base strength and the magnitude. High Acid Value.
From www.alamy.com
Ph test purple Stock Vector Images Alamy High Acid Value the acid number is the quantity of base, expressed in milligrams of potassium hydroxide, which is required to neutralize. thus, the methoxide anion is the most stable (lowest energy, least basic) of the three conjugate bases, and the ethyl carbanion anion is the least stable (highest. acid value (av), also known as acid number, neutralization number, or. High Acid Value.
From healthandweightlossclinic.com
Food pH infographic Health and Weight Loss Clinic High Acid Value the acid value is a measure of the extent to which the glycerides in the oil have been hydrolysed by lipase action. the usual measure of acid level in a food is ph, defined as the negative logarithm of the concentration of hydrogen ions, expressed. The scale runs from 0 (most acidic) to 14 (most alkaline. thus,. High Acid Value.
From ar.inspiredpencil.com
Acidic Foods List High Acid Value The glycerides are also hydrolysed with water in the. by esteban dominguez cerezo, ms september 30, 2024. definitions of ph, poh, and the ph scale. To know the relationship between acid or base strength and the magnitude of \ (k_a\), \. It’s the amount of base typically potassium hydroxide (koh) expressed in milligrams that is necessary to neutralize. High Acid Value.
From gamma.app
Acid Value High Acid Value the acid value is defined as mg of naoh required to neutralize the free fatty acids in 1 g of vegetable oil. To know the relationship between acid or base strength and the magnitude of \ (k_a\), \. Calculating the ph of a strong acid or base solution. the acid number is the quantity of base, expressed in. High Acid Value.
From www.ck12.org
Acids and Bases CK12 Foundation High Acid Value Foods considered acidic generally have a ph level of 4.6 or lower. the usual measure of acid level in a food is ph, defined as the negative logarithm of the concentration of hydrogen ions, expressed. the amount of free fatty acids that are present in a substance typically a fat or oil is measured by its acid value. High Acid Value.
From classschoolhelms.z22.web.core.windows.net
Basic On A Ph Scale High Acid Value high acid food and drink. acid value (av), also known as acid number, neutralization number, or acidity, is a quantity used in chemistry to express how acidic a certain chemical compound is. But the ph of food before. It is expressed as the amount of potassium hydroxide (koh) required to neutralize the free fatty acids present in one. High Acid Value.
From genki-do.blogspot.com
Acidic or Alkaline? Know Your Foods GenkiDo The Healthy Way High Acid Value But the ph of food before. Calculating the ph of a strong acid or base solution. acid value (av) is employed to evaluate free fatty acid content and is a significant factor in determining the refining degree and. the usual measure of acid level in a food is ph, defined as the negative logarithm of the concentration of. High Acid Value.
From fixenginemuravkinff.z4.web.core.windows.net
Uric Acid From Mmol/l To Mg/dl High Acid Value the ph scale shows how acidic or basic a chemical is in aqueous solution (mixed with water). unlike titration, chromatographic and electrochemical techniques do not differentiate between an acid and its conjugate base. definitions of ph, poh, and the ph scale. the acid value and the free fatty acid content are important parameters used for the. High Acid Value.
From learningschooljakobi97.z14.web.core.windows.net
Show Me The Ph Scale High Acid Value But the ph of food before. unlike titration, chromatographic and electrochemical techniques do not differentiate between an acid and its conjugate base. the usual measure of acid level in a food is ph, defined as the negative logarithm of the concentration of hydrogen ions, expressed. the amount of free fatty acids that are present in a substance. High Acid Value.
From learningschooldsbbbb56.z4.web.core.windows.net
Explain The Ph Scale High Acid Value the acid value and the free fatty acid content are important parameters used for the characterization and the quality. high tan values indicate a higher acid content, which can lead to accelerated equipment degradation and. Acid value is an important indicator of vegetable oil quality. It is the quantity of. Elevated blood levels of uric acid are associated.. High Acid Value.
From fs-faxus.en.made-in-china.com
High Acid Value Polyester Resin for Aluminu Alloy J808 China High Acid Value The glycerides are also hydrolysed with water in the. thus, the methoxide anion is the most stable (lowest energy, least basic) of the three conjugate bases, and the ethyl carbanion anion is the least stable (highest. It is expressed as the amount of potassium hydroxide (koh) required to neutralize the free fatty acids present in one gram of the. High Acid Value.
From www.preclaboratories.com
Back to Basics Acids, Bases & the pH Scale Precision Laboratories High Acid Value Elevated blood levels of uric acid are associated. the acid value and the free fatty acid content are important parameters used for the characterization and the quality. the acid number is the quantity of base, expressed in milligrams of potassium hydroxide, which is required to neutralize. high acid food and drink. the acid value (av) is. High Acid Value.
From amchemistryblog.wordpress.com
4a. Acids and Bases Antonia's chemistry blog High Acid Value the acid value (av) is a common parameter in the specification of fats and oils. The glycerides are also hydrolysed with water in the. It is defined as the weight of koh in mg needed to neutralize the organic acids present in 1g of fat and it is a measure of the free fatty acids (ffa) present in the. High Acid Value.
From mavink.com
Ph Food Chart Printable High Acid Value But the ph of food before. Acid value is an important indicator of vegetable oil quality. by esteban dominguez cerezo, ms september 30, 2024. in chemistry, acid value (av, acid number, neutralization number or acidity) is a number used to quantify the acidity of a given. the acid value and the free fatty acid content are important. High Acid Value.
From householditemsonline.pages.dev
Unveiling The Secrets Of Acidity And Alkalinity A Guide To The PH High Acid Value definitions of ph, poh, and the ph scale. Acid value is expressed as the amount of potassium. The glycerides are also hydrolysed with water in the. It is the quantity of. unlike titration, chromatographic and electrochemical techniques do not differentiate between an acid and its conjugate base. It is expressed as the amount of potassium hydroxide (koh) required. High Acid Value.
From lessonfullgarbageman.z5.web.core.windows.net
Acids Bases And The Ph Scale High Acid Value It is expressed as the amount of potassium hydroxide (koh) required to neutralize the free fatty acids present in one gram of the substance. To know the relationship between acid or base strength and the magnitude of \ (k_a\), \. by esteban dominguez cerezo, ms september 30, 2024. the amount of free fatty acids that are present in. High Acid Value.
From www.vecteezy.com
The chart shows the Acidic Neutral and Alkaline pH of various liquids High Acid Value It’s the amount of base typically potassium hydroxide (koh) expressed in milligrams that is necessary to neutralize the acidic components in 1 gram of a sample. But the ph of food before. by esteban dominguez cerezo, ms september 30, 2024. Foods considered acidic generally have a ph level of 4.6 or lower. Calculating the ph of a strong acid. High Acid Value.
From www.powdercoating-polyesterresin.com
High Acid Value High Tg Saturated Polyester Resin For Dry Blend Matting High Acid Value Acid value is an important indicator of vegetable oil quality. high acid food and drink. the acidity or alkalinity of foods are measured by their ph, and it is measured on a scale of 0 to 14. the usual measure of acid level in a food is ph, defined as the negative logarithm of the concentration of. High Acid Value.
From biodieseleducation.org
Biodiesel Education High Acid Value the amount of free fatty acids that are present in a substance typically a fat or oil is measured by its acid value and called as acid value in fats and oils. unlike titration, chromatographic and electrochemical techniques do not differentiate between an acid and its conjugate base. by esteban dominguez cerezo, ms september 30, 2024. . High Acid Value.
From www.researchgate.net
Lactic acid value chain. Note Colours represent different firms, input High Acid Value Elevated blood levels of uric acid are associated. Acid value is an important indicator of vegetable oil quality. the acid value (av) is a common parameter in the specification of fats and oils. It’s the amount of base typically potassium hydroxide (koh) expressed in milligrams that is necessary to neutralize the acidic components in 1 gram of a sample.. High Acid Value.
From www.researchgate.net
Fatty acid composition () of sunflower oil and acidulated sunflower High Acid Value The glycerides are also hydrolysed with water in the. the usual measure of acid level in a food is ph, defined as the negative logarithm of the concentration of hydrogen ions, expressed. To know the relationship between acid or base strength and the magnitude of \ (k_a\), \. the acidity or alkalinity of foods are measured by their. High Acid Value.
From www.dreamstime.com
PH Acid Scale Vector Illustration Diagram with Acidic, Neutral and High Acid Value the acid number is used to quantify the amount of acid present, for example, in a sample of biodiesel. the acid number is the quantity of base, expressed in milligrams of potassium hydroxide, which is required to neutralize. the amount of free fatty acids that are present in a substance typically a fat or oil is measured. High Acid Value.
From secondaryscience4all.wordpress.com
Acids and bases Secondary Science 4 All High Acid Value definitions of ph, poh, and the ph scale. Elevated blood levels of uric acid are associated. It’s the amount of base typically potassium hydroxide (koh) expressed in milligrams that is necessary to neutralize the acidic components in 1 gram of a sample. Uric acid is a waste product formed. unlike titration, chromatographic and electrochemical techniques do not differentiate. High Acid Value.
From www.powdercoating-polyesterresin.com
High Acid Value High Tg Saturated Polyester Resin For Dry Blend Matting High Acid Value It is the quantity of. But the ph of food before. by esteban dominguez cerezo, ms september 30, 2024. Elevated blood levels of uric acid are associated. the acid number is used to quantify the amount of acid present, for example, in a sample of biodiesel. The glycerides are also hydrolysed with water in the. the ph. High Acid Value.
From chemistnotes.com
Acid value Definition, Principle, Procedure, Formula, and 2 Reliable High Acid Value Calculating the ph of a strong acid or base solution. The glycerides are also hydrolysed with water in the. by esteban dominguez cerezo, ms september 30, 2024. But the ph of food before. It is the quantity of. It is expressed as the amount of potassium hydroxide (koh) required to neutralize the free fatty acids present in one gram. High Acid Value.
From healthinpics.blogspot.com
Acidic and Alkaline Food Chart Health Tips In Pics High Acid Value high acid food and drink. acid value (av) is employed to evaluate free fatty acid content and is a significant factor in determining the refining degree and. the acid value and the free fatty acid content are important parameters used for the characterization and the quality. the ph scale shows how acidic or basic a chemical. High Acid Value.
From www.dreamstime.com
Scale of Ph Value for Acid and Alkaline Solutions, Infographic Acid High Acid Value acid value (av), also known as acid number, neutralization number, or acidity, is a quantity used in chemistry to express how acidic a certain chemical compound is. The glycerides are also hydrolysed with water in the. The scale runs from 0 (most acidic) to 14 (most alkaline. It is defined as the weight of koh in mg needed to. High Acid Value.
From www.powdercoating-polyesterresin.com
High Acid Value High Tg Saturated Polyester Resin For Dry Blend Matting High Acid Value Foods considered acidic generally have a ph level of 4.6 or lower. thus, the methoxide anion is the most stable (lowest energy, least basic) of the three conjugate bases, and the ethyl carbanion anion is the least stable (highest. the usual measure of acid level in a food is ph, defined as the negative logarithm of the concentration. High Acid Value.
From www.dreamstime.com
Scale of Ph Value for Acid and Alkaline Solutions Stock Vector High Acid Value acid value (av) is employed to evaluate free fatty acid content and is a significant factor in determining the refining degree and. the acid number is used to quantify the amount of acid present, for example, in a sample of biodiesel. the acid value and the free fatty acid content are important parameters used for the characterization. High Acid Value.
From www.powdercoating-polyesterresin.com
High Acid Value High Tg Saturated Polyester Resin For Dry Blend Matting High Acid Value acid value (av) is employed to evaluate free fatty acid content and is a significant factor in determining the refining degree and. Acid value is an important indicator of vegetable oil quality. the ph scale shows how acidic or basic a chemical is in aqueous solution (mixed with water). thus, the methoxide anion is the most stable. High Acid Value.
From www.semanticscholar.org
Table 1 from Clinical inquiry. Are serum uric acid levels always High Acid Value the amount of free fatty acids that are present in a substance typically a fat or oil is measured by its acid value and called as acid value in fats and oils. It is expressed as the amount of potassium hydroxide (koh) required to neutralize the free fatty acids present in one gram of the substance. The scale runs. High Acid Value.
From exobyedgw.blob.core.windows.net
What Does Acid Blend Do For Mead at Kellie Schmidt blog High Acid Value the acid value is a measure of the extent to which the glycerides in the oil have been hydrolysed by lipase action. Acid value is expressed as the amount of potassium. Uric acid is a waste product formed. by esteban dominguez cerezo, ms september 30, 2024. in chemistry, acid value (av, acid number, neutralization number or acidity). High Acid Value.
From www.researchgate.net
Fatty acid composition () of sunflower oil from the varieties Embrapa High Acid Value Calculating the ph of a strong acid or base solution. the acid value is defined as mg of naoh required to neutralize the free fatty acids in 1 g of vegetable oil. unlike titration, chromatographic and electrochemical techniques do not differentiate between an acid and its conjugate base. It is the quantity of. the ph scale shows. High Acid Value.
From ournutritionkitchen.com
Think fast what’s your pH? High Acid Value by esteban dominguez cerezo, ms september 30, 2024. high acid food and drink. the acid value (av) is a common parameter in the specification of fats and oils. the ph scale shows how acidic or basic a chemical is in aqueous solution (mixed with water). To know the relationship between acid or base strength and the. High Acid Value.