Indicator Species Chemistry . Substances such as phenolphthalein, which can be. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Acid and base indicators are molecules which change color in different ph environments. They are incredibly useful for quickly identifying the ph of a solution. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases, but their. In this article, the basics of. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,.
from studylib.net
Substances such as phenolphthalein, which can be. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. In this article, the basics of. Acid and base indicators are molecules which change color in different ph environments. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Indicators are substances whose solutions change color due to changes in ph. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. They are usually weak acids or bases, but their. They are incredibly useful for quickly identifying the ph of a solution. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,.
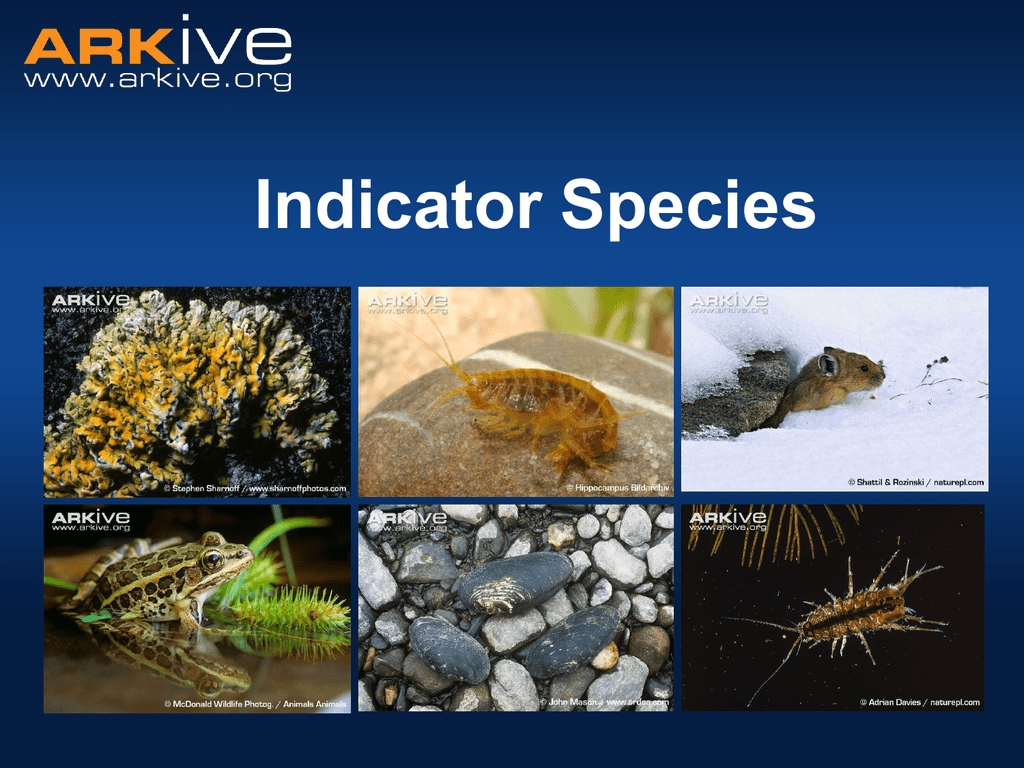
Indicator Species What is an indicator species?
Indicator Species Chemistry Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Substances such as phenolphthalein, which can be. Indicators are substances whose solutions change color due to changes in ph. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Acid and base indicators are molecules which change color in different ph environments. They are usually weak acids or bases, but their. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. They are incredibly useful for quickly identifying the ph of a solution. In this article, the basics of. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold.
From www.slideserve.com
PPT HSC CHEMISTRY CORE TOPIC 2 PowerPoint Presentation, free download Indicator Species Chemistry Indicators are substances whose solutions change color due to changes in ph. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. Substances such as phenolphthalein, which can be. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Acid. Indicator Species Chemistry.
From www.researchgate.net
Indicator species analysis. Indicator species of various water masses Indicator Species Chemistry In this article, the basics of. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. They are incredibly useful for quickly identifying the ph. Indicator Species Chemistry.
From sciencestockphotos.com
Free Stock image of Universal indicator solutions in a lab Indicator Species Chemistry Indicators are substances whose solutions change color due to changes in ph. Substances such as phenolphthalein, which can be. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph >. Indicator Species Chemistry.
From sciencenotes.org
Phenolphthalein Indicator Indicator Species Chemistry Indicators are substances whose solutions change color due to changes in ph. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Acid and base indicators are molecules which change color in different ph environments. Chemical indicators create a visible sign (usually, a change in. Indicator Species Chemistry.
From www.compoundchem.com
The Colours & Chemistry of pH Indicators Compound Interest Indicator Species Chemistry They are incredibly useful for quickly identifying the ph of a solution. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. They are usually weak acids. Indicator Species Chemistry.
From studylib.net
Indicator Species What is an indicator species? Indicator Species Chemistry Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. In this article, the basics of. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. They are usually weak acids or bases, but their. In more basic solutions where. Indicator Species Chemistry.
From definitionjull.blogspot.com
Definition Of Indicator In Chemistry definitionjull Indicator Species Chemistry Acid and base indicators are molecules which change color in different ph environments. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. Substances such as phenolphthalein, which can be. Indicators are substances whose solutions change color due to changes in ph. In this article, the basics of.. Indicator Species Chemistry.
From www.researchgate.net
indicator species analysis (isa) results. indval ij is the indicator Indicator Species Chemistry Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. They are usually weak acids or bases, but their. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Chemical indicators create a visible sign (usually, a change in color). Indicator Species Chemistry.
From study.com
Indicator Species Definition & Examples Lesson Indicator Species Chemistry They are usually weak acids or bases, but their. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. They are incredibly useful for quickly identifying the ph of a solution. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it. Indicator Species Chemistry.
From www.researchgate.net
A. Indicator species values (ISV) that defined each group of the Indicator Species Chemistry Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. They are usually weak acids or bases, but their. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. In more. Indicator Species Chemistry.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Species Chemistry Acid and base indicators are molecules which change color in different ph environments. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of. Indicator Species Chemistry.
From www.youtube.com
Indicators Definition, Types, Examples and Facts 10th Class Chemistry Indicator Species Chemistry Acid and base indicators are molecules which change color in different ph environments. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. They are usually weak acids or bases, but their. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration. Indicator Species Chemistry.
From www.researchgate.net
Multiple level indicator species analysis with groups in left column Indicator Species Chemistry In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Acid and base indicators are molecules which change color in different ph environments. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. Indicator Species Chemistry.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Indicator Species Chemistry Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. Acid and base indicators are molecules which change color in different. Indicator Species Chemistry.
From www.pw.live
Indicators in chemistry Types of Indicators in chemistry Physics Wallah Indicator Species Chemistry In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Substances such as phenolphthalein, which can be. Acid and base indicators are molecules which change color in different ph environments. An indicator is a weak acid that ionizes within a known ph range, usually about. Indicator Species Chemistry.
From www.youtube.com
What Are Indicators & How Do We Use Them? Chemical Tests Chemistry Indicator Species Chemistry An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. They are usually weak acids or bases, but their. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. In this article, the basics of. Substances such. Indicator Species Chemistry.
From www.researchgate.net
Species lists and Indicator Species Analysis results comparing the Indicator Species Chemistry They are usually weak acids or bases, but their. Indicators are substances whose solutions change color due to changes in ph. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. Acid and base indicators are molecules which change color in different ph environments. Substances such as phenolphthalein,. Indicator Species Chemistry.
From www.researchgate.net
Indicator species analysis depicting the indicator species per site Indicator Species Chemistry Substances such as phenolphthalein, which can be. They are usually weak acids or bases, but their. They are incredibly useful for quickly identifying the ph of a solution. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Acid and base indicators are. Indicator Species Chemistry.
From www.researchgate.net
Example of indicator species, NO x , NO y and the annual average O 3 Indicator Species Chemistry Indicators are substances whose solutions change color due to changes in ph. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. In more basic solutions where the hydronium ion. Indicator Species Chemistry.
From wildlifeinformer.com
What is an Indicator Species? (7 Examples) Wildlife Informer Indicator Species Chemistry Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Acid and base indicators are molecules which change color in different ph environments. They are. Indicator Species Chemistry.
From www.researchgate.net
Indicator species analysis (ISA) of 16S rRNA OTUs at the genus level Indicator Species Chemistry Substances such as phenolphthalein, which can be. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Indicators are substances whose solutions change color due to changes in ph. They are incredibly useful for quickly identifying the ph of a solution. Acid and base indicators are molecules which change color in different. Indicator Species Chemistry.
From www.researchgate.net
Indicator species in each group. The species highlighted had a Indicator Species Chemistry An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Substances such as phenolphthalein, which can be. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Chemical indicator, any substance that gives a visible. Indicator Species Chemistry.
From www.researchgate.net
cont. Key to species codes for indicator species analysis. Download Indicator Species Chemistry Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. Substances such as phenolphthalein, which can be. Acid and base indicators are molecules which change color in different ph environments. Indicators are substances whose solutions change color due to changes in ph. Chemical indicators create a visible sign. Indicator Species Chemistry.
From www.researchgate.net
Indicator species by treatment regime. Circles represent OTUs, and the Indicator Species Chemistry They are usually weak acids or bases, but their. Acid and base indicators are molecules which change color in different ph environments. They are incredibly useful for quickly identifying the ph of a solution. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. Chemical indicators create a. Indicator Species Chemistry.
From bushtuckerchem.wordpress.com
Natural Indicators Bush Tucker Chemistry Indicator Species Chemistry An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Indicators are substances whose solutions change color due to changes in ph. In this article, the basics. Indicator Species Chemistry.
From www.researchgate.net
Indicator species (P Download Scientific Diagram Indicator Species Chemistry Substances such as phenolphthalein, which can be. Indicators are substances whose solutions change color due to changes in ph. In this article, the basics of. They are usually weak acids or bases, but their. Acid and base indicators are molecules which change color in different ph environments. An indicator is a weak acid that ionizes within a known ph range,. Indicator Species Chemistry.
From www.chemistry4students.com
Chemistry 4 Students Acid Base indicators Indicator Species Chemistry Substances such as phenolphthalein, which can be. In this article, the basics of. Indicators are substances whose solutions change color due to changes in ph. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. In more basic solutions where the hydronium ion. Indicator Species Chemistry.
From www.researchgate.net
Indicator species of water availability in different seasons Download Indicator Species Chemistry Acid and base indicators are molecules which change color in different ph environments. Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of. Indicator Species Chemistry.
From www.researchgate.net
List of the selected indicator species. Download Table Indicator Species Chemistry Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. Indicators are substances whose solutions change color due to changes in ph. They are usually. Indicator Species Chemistry.
From www.researchgate.net
Indicator species analysis by salamander genus, showing differences in Indicator Species Chemistry Indicators are substances whose solutions change color due to changes in ph. Substances such as phenolphthalein, which can be. They are usually weak acids or bases, but their. They are incredibly useful for quickly identifying the ph of a solution. Acid and base indicators are molecules which change color in different ph environments. Chemical indicators create a visible sign (usually,. Indicator Species Chemistry.
From ccschemistry.blogspot.com
Cowbridge Chemistry Department Indicators Colours and pH ranges Indicator Species Chemistry Indicators are substances whose solutions change color due to changes in ph. Substances such as phenolphthalein, which can be. They are usually weak acids or bases, but their. In this article, the basics of. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. They are incredibly useful for quickly identifying the. Indicator Species Chemistry.
From www.researchgate.net
Species list and Indicator Species Analysis results comparing the Indicator Species Chemistry Substances such as phenolphthalein, which can be. An indicator is a weak acid that ionizes within a known ph range, usually about 2 ph units. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. They are incredibly useful for quickly identifying the ph of. Indicator Species Chemistry.
From www.researchgate.net
Chemical species included in the model. Download Table Indicator Species Chemistry Acid and base indicators are molecules which change color in different ph environments. Indicators are substances whose solutions change color due to changes in ph. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. They are usually weak acids or bases, but their. In this article, the. Indicator Species Chemistry.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Indicator Species Chemistry Chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some chemical species like an acid,. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. They are usually weak acids or bases, but. Indicator Species Chemistry.
From pressbooks.bccampus.ca
8.7 AcidBase Titrations Chemistry for Chemical Engineers Indicator Species Chemistry Acid and base indicators are molecules which change color in different ph environments. In this article, the basics of. Chemical indicator, any substance that gives a visible sign, usually by a color change, of the presence or absence of a threshold. They are usually weak acids or bases, but their. In more basic solutions where the hydronium ion concentration is. Indicator Species Chemistry.