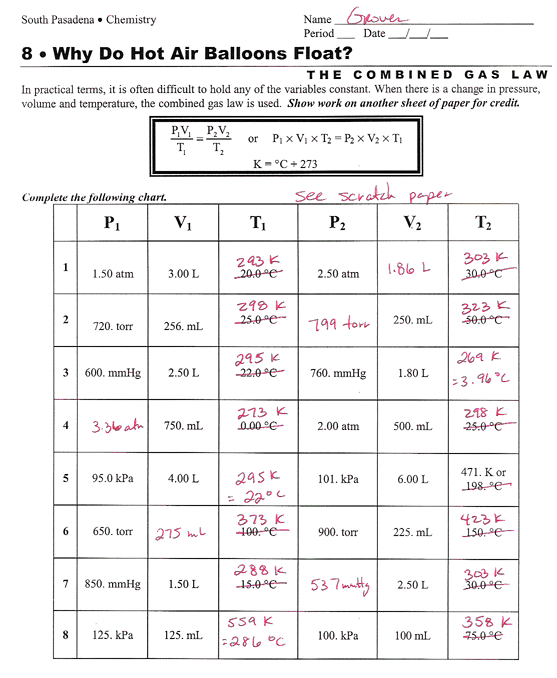
Mixed Gas Laws Worksheet With Answers . Write the form of the gas law you plan to use to. As always, include enough work and show the units to ensure full credit. The combined gas law key solve the following problems. What is the volume at 10 mm hg at the same temperature? Show all work for all problems. A sample of neon gas occupies a volume of 752 ml at 250c. Graham’s law and dalton’s law of partial pressures. A constant volume of oxygen is. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. What volume will the gas occupy at. What will its new pressure be? How fast would a mole of. A gas occupies 3.5l at 2.5 mm hg pressure. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? Examine each question and then.
from materialcampusrosefish.z21.web.core.windows.net
What is the volume at 10 mm hg at the same temperature? A sample of neon gas occupies a volume of 752 ml at 250c. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. As always, include enough work and show the units to ensure full credit. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? Graham’s law and dalton’s law of partial pressures. A constant volume of oxygen is. Write the form of the gas law you plan to use to. The combined gas law key solve the following problems. A gas occupies 3.5l at 2.5 mm hg pressure.
Combined Gas Law Worksheet With Answers
Mixed Gas Laws Worksheet With Answers Graham’s law and dalton’s law of partial pressures. What volume will the gas occupy at. Show all work for all problems. What will its new pressure be? 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? A sample of neon gas occupies a volume of 752 ml at 250c. Write the form of the gas law you plan to use to. How fast would a mole of. A constant volume of oxygen is. A gas occupies 3.5l at 2.5 mm hg pressure. What is the volume at 10 mm hg at the same temperature? As always, include enough work and show the units to ensure full credit. The combined gas law key solve the following problems. Graham’s law and dalton’s law of partial pressures. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. Examine each question and then.
From printablefullstria.z19.web.core.windows.net
Gas Laws Worksheet With Answers Mixed Gas Laws Worksheet With Answers Examine each question and then. Show all work for all problems. A gas occupies 3.5l at 2.5 mm hg pressure. Graham’s law and dalton’s law of partial pressures. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. 1) how many moles. Mixed Gas Laws Worksheet With Answers.
From www.scribd.com
Mixed Gas Law Worksheet PDF Mixed Gas Laws Worksheet With Answers A constant volume of oxygen is. A sample of neon gas occupies a volume of 752 ml at 250c. Examine each question and then. What is the volume at 10 mm hg at the same temperature? Write the form of the gas law you plan to use to. Graham’s law and dalton’s law of partial pressures. A gas occupies 3.5l. Mixed Gas Laws Worksheet With Answers.
From www.studypool.com
SOLUTION Gas laws key worksheet with answer Studypool Mixed Gas Laws Worksheet With Answers Show all work for all problems. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? What volume will the gas occupy at. The combined gas law key solve the following problems. Graham’s law and dalton’s law of partial pressures. A sample of neon gas occupies a volume of. Mixed Gas Laws Worksheet With Answers.
From studydblang99.z21.web.core.windows.net
Mixed Gas Laws Worksheet Answers Mixed Gas Laws Worksheet With Answers Examine each question and then. A sample of neon gas occupies a volume of 752 ml at 250c. How fast would a mole of. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? As always, include enough work and show the units to ensure full credit. What will. Mixed Gas Laws Worksheet With Answers.
From myans.bhantedhammika.net
Tips For Completing Combined Gas Law Worksheet 1 Answer Key Mixed Gas Laws Worksheet With Answers A constant volume of oxygen is. What is the volume at 10 mm hg at the same temperature? The combined gas law key solve the following problems. What will its new pressure be? A gas occupies 3.5l at 2.5 mm hg pressure. What volume will the gas occupy at. A sample of neon gas occupies a volume of 752 ml. Mixed Gas Laws Worksheet With Answers.
From www.studocu.com
Mixed Gas Laws Worksheet Key Mechanical Engineering Studocu Mixed Gas Laws Worksheet With Answers What is the volume at 10 mm hg at the same temperature? What will its new pressure be? A constant volume of oxygen is. As always, include enough work and show the units to ensure full credit. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? Graham’s law. Mixed Gas Laws Worksheet With Answers.
From www.scribd.com
Mixed gas law worksheet key PDF Mixed Gas Laws Worksheet With Answers What is the volume at 10 mm hg at the same temperature? Show all work for all problems. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. A constant volume of oxygen is. How fast would a mole of. As always,. Mixed Gas Laws Worksheet With Answers.
From www.worksheeto.com
8 Combined Gas Law Worksheet / Mixed Gas Laws Worksheet With Answers How fast would a mole of. Show all work for all problems. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? Examine each question and then. What is the volume at 10 mm hg at the same temperature? 1) if i initially have 4.0 l of a gas. Mixed Gas Laws Worksheet With Answers.
From lessondbostermann.z19.web.core.windows.net
The Gas Laws Worksheet Answers Mixed Gas Laws Worksheet With Answers The combined gas law key solve the following problems. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. What is the volume at 10 mm hg at the same temperature? A sample of neon gas occupies a volume of 752 ml. Mixed Gas Laws Worksheet With Answers.
From worksheets.decoomo.com
30++ Mixed Gas Laws Worksheet Answers Worksheets Decoomo Mixed Gas Laws Worksheet With Answers As always, include enough work and show the units to ensure full credit. Write the form of the gas law you plan to use to. A sample of neon gas occupies a volume of 752 ml at 250c. Show all work for all problems. A constant volume of oxygen is. What volume will the gas occupy at. The combined gas. Mixed Gas Laws Worksheet With Answers.
From studylib.net
GAS LAW WORKSHEET Mixed Gas Laws, including Ideal Gas Law Mixed Gas Laws Worksheet With Answers What is the volume at 10 mm hg at the same temperature? A constant volume of oxygen is. What volume will the gas occupy at. Graham’s law and dalton’s law of partial pressures. Show all work for all problems. A gas occupies 3.5l at 2.5 mm hg pressure. 1) how many moles of gas occupy 98 l at a pressure. Mixed Gas Laws Worksheet With Answers.
From printablezonececilia.z19.web.core.windows.net
Gas Laws Worksheet Answers Mixed Gas Laws Worksheet With Answers What will its new pressure be? Write the form of the gas law you plan to use to. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? Show all work for all problems. Examine each question and then. The combined gas law key solve the following problems. A. Mixed Gas Laws Worksheet With Answers.
From www.worksheeto.com
17 Mixed Gas Laws Worksheet Answers / Mixed Gas Laws Worksheet With Answers 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? How fast would a mole of. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. Write the form of the. Mixed Gas Laws Worksheet With Answers.
From worksheetzone.org
Mixed Gas Laws Worksheet Worksheet Mixed Gas Laws Worksheet With Answers What will its new pressure be? The combined gas law key solve the following problems. How fast would a mole of. Examine each question and then. A constant volume of oxygen is. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4.. Mixed Gas Laws Worksheet With Answers.
From animalia-life.club
Combined Gas Law Worksheet Answers Mixed Gas Laws Worksheet With Answers Show all work for all problems. What volume will the gas occupy at. As always, include enough work and show the units to ensure full credit. Write the form of the gas law you plan to use to. A gas occupies 3.5l at 2.5 mm hg pressure. What will its new pressure be? 1) how many moles of gas occupy. Mixed Gas Laws Worksheet With Answers.
From www.studypool.com
SOLUTION Ideal gas law worksheet 2 answer Studypool Mixed Gas Laws Worksheet With Answers How fast would a mole of. Graham’s law and dalton’s law of partial pressures. A constant volume of oxygen is. Show all work for all problems. A sample of neon gas occupies a volume of 752 ml at 250c. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be. Mixed Gas Laws Worksheet With Answers.
From studylib.net
mixed gas laws worksheet solutions Mixed Gas Laws Worksheet With Answers The combined gas law key solve the following problems. A gas occupies 3.5l at 2.5 mm hg pressure. A constant volume of oxygen is. What volume will the gas occupy at. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? As always, include enough work and show the. Mixed Gas Laws Worksheet With Answers.
From www.studocu.com
Mixed Gas Laws Worksheet Grey Mixed Gas Laws Worksheet How many moles Mixed Gas Laws Worksheet With Answers The combined gas law key solve the following problems. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? Examine each question and then. A sample of neon gas occupies a volume of 752 ml at 250c. As always, include enough work and show the units to ensure full. Mixed Gas Laws Worksheet With Answers.
From materialcampusrosefish.z21.web.core.windows.net
Combined Gas Law Worksheet With Answers Mixed Gas Laws Worksheet With Answers As always, include enough work and show the units to ensure full credit. A gas occupies 3.5l at 2.5 mm hg pressure. The combined gas law key solve the following problems. How fast would a mole of. What is the volume at 10 mm hg at the same temperature? Show all work for all problems. What volume will the gas. Mixed Gas Laws Worksheet With Answers.
From learninglisttheiss.z21.web.core.windows.net
Understanding Gas Laws Worksheet Answers Mixed Gas Laws Worksheet With Answers A gas occupies 3.5l at 2.5 mm hg pressure. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. A constant volume of oxygen is. As always, include enough work and show the units to ensure full credit. How fast would a. Mixed Gas Laws Worksheet With Answers.
From studylane55.z19.web.core.windows.net
Gas Laws Practice Worksheet Mixed Gas Laws Worksheet With Answers A constant volume of oxygen is. Write the form of the gas law you plan to use to. As always, include enough work and show the units to ensure full credit. A gas occupies 3.5l at 2.5 mm hg pressure. What is the volume at 10 mm hg at the same temperature? The combined gas law key solve the following. Mixed Gas Laws Worksheet With Answers.
From davida.davivienda.com
Worksheet Boyle's Law And Charles Law Printable Word Searches Mixed Gas Laws Worksheet With Answers What will its new pressure be? Examine each question and then. What volume will the gas occupy at. What is the volume at 10 mm hg at the same temperature? Show all work for all problems. A sample of neon gas occupies a volume of 752 ml at 250c. Graham’s law and dalton’s law of partial pressures. How fast would. Mixed Gas Laws Worksheet With Answers.
From www.studocu.com
Mixed Gas Laws Worksheet noon the temperature has risen to 45 0 C Mixed Gas Laws Worksheet With Answers The combined gas law key solve the following problems. What will its new pressure be? Examine each question and then. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. 1) how many moles of gas occupy 98 l at a pressure. Mixed Gas Laws Worksheet With Answers.
From printablefullminis.z13.web.core.windows.net
Gas Laws Worksheet With Answers Mixed Gas Laws Worksheet With Answers Write the form of the gas law you plan to use to. What volume will the gas occupy at. Graham’s law and dalton’s law of partial pressures. How fast would a mole of. What will its new pressure be? As always, include enough work and show the units to ensure full credit. The combined gas law key solve the following. Mixed Gas Laws Worksheet With Answers.
From studylib.net
Mixed Gas Law Worksheet Answers available at end Name (show Mixed Gas Laws Worksheet With Answers Graham’s law and dalton’s law of partial pressures. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? Examine each question and then. A constant volume of oxygen is. What is the volume at 10 mm hg at the same temperature? What will its new pressure be? How fast. Mixed Gas Laws Worksheet With Answers.
From studylib.net
MIXED GAS LAWS WORKSHEET Mixed Gas Laws Worksheet With Answers As always, include enough work and show the units to ensure full credit. Examine each question and then. A sample of neon gas occupies a volume of 752 ml at 250c. A gas occupies 3.5l at 2.5 mm hg pressure. A constant volume of oxygen is. Show all work for all problems. 1) if i initially have 4.0 l of. Mixed Gas Laws Worksheet With Answers.
From davida.davivienda.com
Combined Gas Law Worksheet Answers Printable Word Searches Mixed Gas Laws Worksheet With Answers 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? The combined gas law key solve the following problems. What volume will the gas occupy at. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i. Mixed Gas Laws Worksheet With Answers.
From www.studypool.com
SOLUTION Gas laws key worksheet with answer Studypool Mixed Gas Laws Worksheet With Answers What is the volume at 10 mm hg at the same temperature? A gas occupies 3.5l at 2.5 mm hg pressure. How fast would a mole of. What volume will the gas occupy at. What will its new pressure be? 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292. Mixed Gas Laws Worksheet With Answers.
From www.worksheeto.com
17 Mixed Gas Laws Worksheet Answers / Mixed Gas Laws Worksheet With Answers 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? Write the form of the gas law you plan to use to. The combined gas law key solve the following problems. As always, include enough work and show the units to ensure full credit. Examine each question and then.. Mixed Gas Laws Worksheet With Answers.
From animalia-life.club
Combined Gas Law Worksheet Answers Mixed Gas Laws Worksheet With Answers Graham’s law and dalton’s law of partial pressures. Show all work for all problems. A gas occupies 3.5l at 2.5 mm hg pressure. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. What is the volume at 10 mm hg at. Mixed Gas Laws Worksheet With Answers.
From lessonmagicfootfall.z14.web.core.windows.net
Understanding Gas Laws Worksheet Answers Mixed Gas Laws Worksheet With Answers The combined gas law key solve the following problems. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. How fast would a mole of. A sample of neon gas occupies a volume of 752 ml at 250c. A constant volume of. Mixed Gas Laws Worksheet With Answers.
From www.scribd.com
Mixed Gas Laws Worksheet With Answer PDF Gases Atmosphere Of Earth Mixed Gas Laws Worksheet With Answers Write the form of the gas law you plan to use to. How fast would a mole of. A gas occupies 3.5l at 2.5 mm hg pressure. 1) if i initially have 4.0 l of a gas at a pressure of 1.1 atm, what will the volume be if i increase the pressure to 3.4. Graham’s law and dalton’s law. Mixed Gas Laws Worksheet With Answers.
From www.studypool.com
SOLUTION 10chap mixed gas laws worksheet Studypool Mixed Gas Laws Worksheet With Answers How fast would a mole of. What will its new pressure be? A sample of neon gas occupies a volume of 752 ml at 250c. A constant volume of oxygen is. A gas occupies 3.5l at 2.5 mm hg pressure. Examine each question and then. 1) if i initially have 4.0 l of a gas at a pressure of 1.1. Mixed Gas Laws Worksheet With Answers.
From obropolox.blogspot.com
40 chemistry gas laws worksheet with work Worksheet Resource Mixed Gas Laws Worksheet With Answers What will its new pressure be? What volume will the gas occupy at. Examine each question and then. What is the volume at 10 mm hg at the same temperature? Show all work for all problems. The combined gas law key solve the following problems. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres. Mixed Gas Laws Worksheet With Answers.
From studyzoneohsinevitably.z13.web.core.windows.net
Mixed Gas Law Worksheets Mixed Gas Laws Worksheet With Answers A gas occupies 3.5l at 2.5 mm hg pressure. Show all work for all problems. 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? The combined gas law key solve the following problems. A constant volume of oxygen is. As always, include enough work and show the units. Mixed Gas Laws Worksheet With Answers.