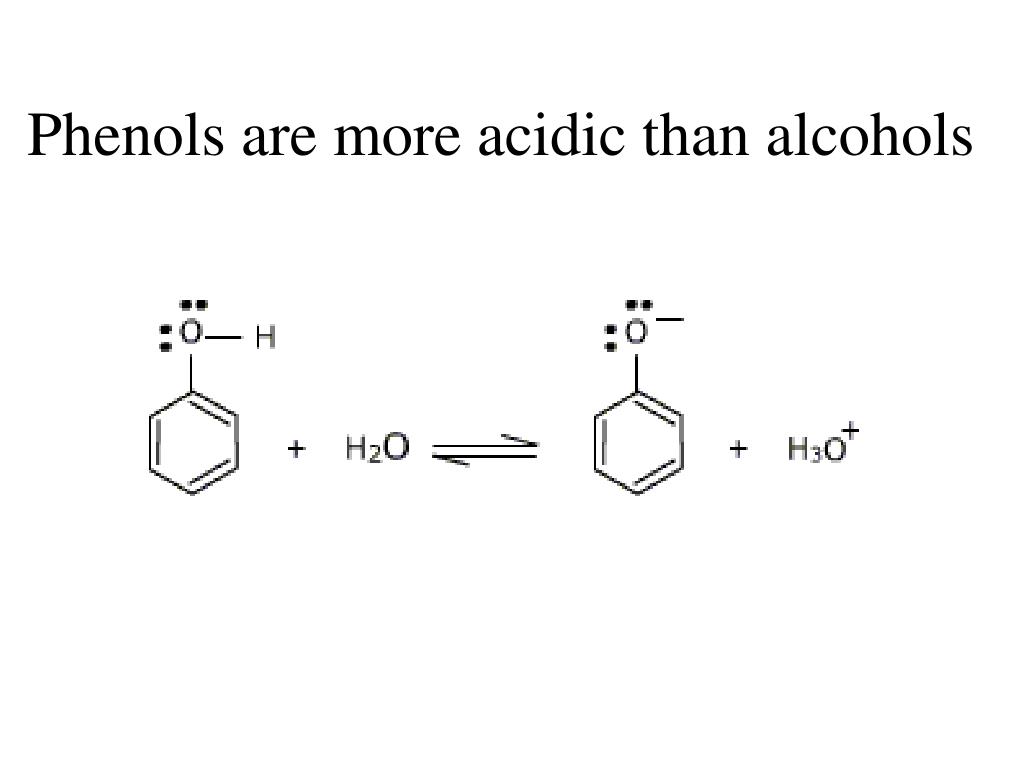
What Is Phenol Much More Acidic Than Alcohol . Phenols are about a million times more acidic than alcohols. Explain the difference in acidity between two given alcohols or phenols. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. Explain why phenols are more acidic than alcohols. The nitration of phenol with concentrated nitric acid. That means that a very dilute solution isn't really acidic enough to turn litmus. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution.
from www.slideserve.com
In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. The nitration of phenol with concentrated nitric acid. Explain the difference in acidity between two given alcohols or phenols. That means that a very dilute solution isn't really acidic enough to turn litmus. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. Phenols are about a million times more acidic than alcohols. Explain why phenols are more acidic than alcohols. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide.
PPT Alcohols PowerPoint Presentation, free download ID4396111
What Is Phenol Much More Acidic Than Alcohol Explain why phenols are more acidic than alcohols. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Explain why phenols are more acidic than alcohols. That means that a very dilute solution isn't really acidic enough to turn litmus. Phenols are about a million times more acidic than alcohols. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. Explain the difference in acidity between two given alcohols or phenols. The nitration of phenol with concentrated nitric acid. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution.
From www.youtube.com
Phenols are more acidic than alcohals.why YouTube What Is Phenol Much More Acidic Than Alcohol Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. That means that a very dilute solution isn't really acidic enough to turn litmus. Explain the difference in acidity between two given alcohols or phenols. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide.. What Is Phenol Much More Acidic Than Alcohol.
From byjus.com
Phenols are more acidic than alcohol. Explain why? What Is Phenol Much More Acidic Than Alcohol They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. The nitration of phenol with concentrated nitric acid. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. Explain the difference in. What Is Phenol Much More Acidic Than Alcohol.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in What Is Phenol Much More Acidic Than Alcohol Phenols are about a million times more acidic than alcohols. That means that a very dilute solution isn't really acidic enough to turn litmus. Explain why phenols are more acidic than alcohols. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. They. What Is Phenol Much More Acidic Than Alcohol.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID What Is Phenol Much More Acidic Than Alcohol The nitration of phenol with concentrated nitric acid. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Explain why phenols. What Is Phenol Much More Acidic Than Alcohol.
From www.pearson.com
Identify the most acidic phenol Pearson+ Channels What Is Phenol Much More Acidic Than Alcohol The nitration of phenol with concentrated nitric acid. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Explain the difference in acidity between two given alcohols or phenols. Explain why phenols are more acidic than alcohols. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium. What Is Phenol Much More Acidic Than Alcohol.
From www.youtube.com
WHY PHENOLS ARE MORE ACIDIC THAN ALCOHOLS Amazing Chemistry By Mandeep What Is Phenol Much More Acidic Than Alcohol Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. The nitration of phenol with concentrated nitric acid. Phenols are about a million times more acidic than. What Is Phenol Much More Acidic Than Alcohol.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID4524523 What Is Phenol Much More Acidic Than Alcohol They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Explain the difference. What Is Phenol Much More Acidic Than Alcohol.
From www.youtube.com
15) Acidic nature of phenol Why Phenol is more acidic than Alcohol What Is Phenol Much More Acidic Than Alcohol Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. The nitration of phenol with concentrated nitric acid. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Explain why phenols are more acidic than alcohols. In aqueous solutions, phenols are weakly acidic and lower the. What Is Phenol Much More Acidic Than Alcohol.
From www.youtube.com
Acidic nature phenols and compare with that of alcohols/acidic nature What Is Phenol Much More Acidic Than Alcohol Explain why phenols are more acidic than alcohols. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. The nitration of phenol. What Is Phenol Much More Acidic Than Alcohol.
From www.youtube.com
Structure of Phenol II Phenol is more acidic than alcohol?II WhyPhenol What Is Phenol Much More Acidic Than Alcohol Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. Explain the difference in acidity between two given alcohols or phenols. Explain why phenols are more acidic than alcohols. That means that a very dilute solution isn't really acidic enough to turn litmus. The nitration of phenol with. What Is Phenol Much More Acidic Than Alcohol.
From slideplayer.com
Chapter 17 Alcohols and Phenols ppt download What Is Phenol Much More Acidic Than Alcohol That means that a very dilute solution isn't really acidic enough to turn litmus. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. Phenols are about a million times more acidic than alcohols. Explain the difference in acidity between two given alcohols or phenols. Phenol is weakly. What Is Phenol Much More Acidic Than Alcohol.
From byjus.com
Phenols are more acidic than alcohol. Explain why? What Is Phenol Much More Acidic Than Alcohol Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. The nitration of phenol with concentrated nitric acid. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. That means that a very dilute solution isn't really acidic enough to turn litmus. Phenol is more acidic than ethanol because in phenol lone pair. What Is Phenol Much More Acidic Than Alcohol.
From www.slideserve.com
PPT Alcohols PowerPoint Presentation, free download ID4396111 What Is Phenol Much More Acidic Than Alcohol Phenols are about a million times more acidic than alcohols. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Explain why phenols are more acidic than alcohols. That means that a very dilute solution isn't really acidic enough to turn litmus. The. What Is Phenol Much More Acidic Than Alcohol.
From www.youtube.com
Difference Between Alcohol And Phenol?Class Series YouTube What Is Phenol Much More Acidic Than Alcohol Explain the difference in acidity between two given alcohols or phenols. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. They are therefore soluble in dilute aqueous naoh and can often be separated. What Is Phenol Much More Acidic Than Alcohol.
From www.slideserve.com
PPT Organic Chemistry II Alcohols, Phenols, Thiols , Ethers, and What Is Phenol Much More Acidic Than Alcohol Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. The nitration of phenol with concentrated nitric acid. Explain the difference in acidity between two given alcohols or phenols. Phenols are about a million times more acidic than alcohols. That means that a very dilute solution isn't really. What Is Phenol Much More Acidic Than Alcohol.
From brainly.in
Give reaason why phenol is more acidic than methanol Brainly.in What Is Phenol Much More Acidic Than Alcohol Phenols are about a million times more acidic than alcohols. The nitration of phenol with concentrated nitric acid. That means that a very dilute solution isn't really acidic enough to turn litmus. Explain the difference in acidity between two given alcohols or phenols. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance. What Is Phenol Much More Acidic Than Alcohol.
From www.youtube.com
Phenol is more acidic than alcohol...why?... YouTube What Is Phenol Much More Acidic Than Alcohol They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. The nitration of phenol with concentrated nitric acid. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. That means that a very dilute solution isn't really acidic enough to turn litmus. Explain why phenols are. What Is Phenol Much More Acidic Than Alcohol.
From www.youtube.com
Comment Carboxylic acid is More acidic than phenol and alcohol YouTube What Is Phenol Much More Acidic Than Alcohol Phenols are about a million times more acidic than alcohols. The nitration of phenol with concentrated nitric acid. Explain why phenols are more acidic than alcohols. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. That means that a very dilute solution isn't really acidic enough to turn litmus. Phenol is more acidic than ethanol because. What Is Phenol Much More Acidic Than Alcohol.
From sciencebyjus.blogspot.com
Acidic Nature of Phenols? Why phenols are more acidic than alcohol What Is Phenol Much More Acidic Than Alcohol Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. That means that a very dilute solution isn't really acidic enough to turn litmus. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic. What Is Phenol Much More Acidic Than Alcohol.
From byjus.com
Phenol is having more acidic character than alcohol. Explain why? What Is Phenol Much More Acidic Than Alcohol That means that a very dilute solution isn't really acidic enough to turn litmus. Explain the difference in acidity between two given alcohols or phenols. The nitration of phenol with concentrated nitric acid. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. In aqueous solutions, phenols are weakly. What Is Phenol Much More Acidic Than Alcohol.
From www.slideserve.com
PPT Phenols PowerPoint Presentation ID735552 What Is Phenol Much More Acidic Than Alcohol That means that a very dilute solution isn't really acidic enough to turn litmus. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. The nitration of phenol with concentrated nitric acid. Explain why phenols are. What Is Phenol Much More Acidic Than Alcohol.
From chemizi.blogspot.com
Phenol is acidic or basic and Which is stronger acid phenol or cresol What Is Phenol Much More Acidic Than Alcohol Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. That means that a very dilute solution isn't really acidic enough to turn litmus. Phenols are about a million times more acidic than alcohols. Explain why phenols are more acidic than alcohols. The nitration of phenol with concentrated. What Is Phenol Much More Acidic Than Alcohol.
From www.youtube.com
Why Carboxylic acid are more acidic than Phenol & Alcohol 45 Class What Is Phenol Much More Acidic Than Alcohol They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. The nitration of phenol with concentrated nitric acid. Explain why phenols are more acidic than alcohols. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. Explain the difference in acidity between two given alcohols or. What Is Phenol Much More Acidic Than Alcohol.
From www.slideserve.com
PPT Alcohols PowerPoint Presentation, free download ID6508687 What Is Phenol Much More Acidic Than Alcohol Explain why phenols are more acidic than alcohols. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. That means that a very dilute solution isn't really acidic enough to turn litmus. In aqueous solutions,. What Is Phenol Much More Acidic Than Alcohol.
From www.doubtnut.com
Carboxylic acids are more acidic than phenols and alchols. Explin What Is Phenol Much More Acidic Than Alcohol Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. That means that a very dilute solution isn't really acidic enough to turn litmus. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Explain the difference in acidity between two given alcohols or phenols. Phenols. What Is Phenol Much More Acidic Than Alcohol.
From www.youtube.com
why Phenol is More acidic than alcohol ? फिनोल अल्कोहल से ज्यादा एसिडिक What Is Phenol Much More Acidic Than Alcohol They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. That means that a very dilute solution isn't really acidic enough to turn litmus. Explain the difference in acidity between two given alcohols or phenols. Explain why phenols are more acidic than alcohols. In aqueous solutions, phenols are weakly. What Is Phenol Much More Acidic Than Alcohol.
From www.youtube.com
The correct order of relative acidic strength of phenol, ethyl alcohol What Is Phenol Much More Acidic Than Alcohol Explain why phenols are more acidic than alcohols. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. That means that a very dilute solution isn't really acidic enough to turn litmus. Phenol is more. What Is Phenol Much More Acidic Than Alcohol.
From www.doubtnut.com
Phenol is more acidic than alcohol because What Is Phenol Much More Acidic Than Alcohol Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Explain the difference in acidity between two given alcohols or phenols. Phenols are about a million times more acidic than alcohols. Phenol is more acidic than. What Is Phenol Much More Acidic Than Alcohol.
From www.youtube.com
Phenol is More Acidic than Ethanol Alcohols, Phenols and Ethers What Is Phenol Much More Acidic Than Alcohol That means that a very dilute solution isn't really acidic enough to turn litmus. Explain why phenols are more acidic than alcohols. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. The nitration of. What Is Phenol Much More Acidic Than Alcohol.
From www.pinterest.com
Why phenols are stronger acids than alcohols. Organic chemistry What Is Phenol Much More Acidic Than Alcohol Explain why phenols are more acidic than alcohols. Explain the difference in acidity between two given alcohols or phenols. That means that a very dilute solution isn't really acidic enough to turn litmus. Phenols are about a million times more acidic than alcohols. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Phenol is weakly. What Is Phenol Much More Acidic Than Alcohol.
From pediaa.com
Difference Between Phenol and Benzoic Acid Definition, Structure What Is Phenol Much More Acidic Than Alcohol Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. The nitration of phenol with concentrated nitric acid. Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. Explain why phenols. What Is Phenol Much More Acidic Than Alcohol.
From www.toppr.com
Phenol are more acidic than alcohols. Why? What Is Phenol Much More Acidic Than Alcohol Phenol is weakly acidic, but stronger than alcohols, and reacts with sodium hydroxide. The nitration of phenol with concentrated nitric acid. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. Explain the difference. What Is Phenol Much More Acidic Than Alcohol.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in What Is Phenol Much More Acidic Than Alcohol Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. Explain why phenols are more acidic than alcohols. That means that a very dilute solution isn't really acidic enough to turn litmus. Explain the difference in acidity between two given alcohols or phenols. In aqueous solutions, phenols are. What Is Phenol Much More Acidic Than Alcohol.
From www.doubtnut.com
Why is phenol more acidic than ethanol What Is Phenol Much More Acidic Than Alcohol They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply by basic extraction into. Phenol is more acidic than ethanol because in phenol lone pair of electrons are utilized in resonance stabilization so bond length between. In aqueous solutions, phenols are weakly acidic and lower the ph of a solution. The nitration of. What Is Phenol Much More Acidic Than Alcohol.
From www.slideserve.com
PPT Alcohols PowerPoint Presentation, free download ID3982776 What Is Phenol Much More Acidic Than Alcohol Phenols are about a million times more acidic than alcohols. That means that a very dilute solution isn't really acidic enough to turn litmus. Explain the difference in acidity between two given alcohols or phenols. Explain why phenols are more acidic than alcohols. They are therefore soluble in dilute aqueous naoh and can often be separated from a mixture simply. What Is Phenol Much More Acidic Than Alcohol.