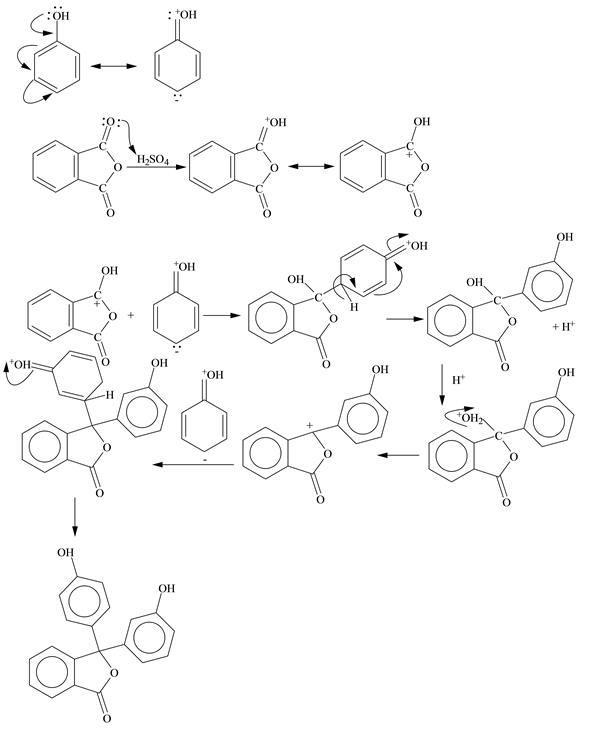
Phenolphthalein Indicator Reaction Equation . At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. It has a chemical formula c 20 h 14 o 4. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein, whose structure is shown. It is written as “phph” or hin”.
from www.vedantu.com
At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. Phenolphthalein, whose structure is shown. In this case, the weak. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. It has a chemical formula c 20 h 14 o 4. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. In this case, the weak acid is colourless and its ion is bright pink. It is written as “phph” or hin”.
How will you convert phenol to phenolphthalein?
Phenolphthalein Indicator Reaction Equation In this case, the weak. It is written as “phph” or hin”. Phenolphthalein, whose structure is shown. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. In this case, the weak acid is colourless and its ion is bright pink. In this case, the weak. It has a chemical formula c 20 h 14 o 4. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. As an indicator it turns pink to red in alkaline and is colourless in acid solutions.
From chemistry.stackexchange.com
organic chemistry Why does phenolphthalein form in this reaction Phenolphthalein Indicator Reaction Equation Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein, whose structure is shown.. Phenolphthalein Indicator Reaction Equation.
From www.chegg.com
Solved Draw a curly arrow mechanism for the reaction of Phenolphthalein Indicator Reaction Equation It is written as “phph” or hin”. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein, whose structure is shown. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case,. Phenolphthalein Indicator Reaction Equation.
From www.chegg.com
Chemistry Archive February 15, 2017 Phenolphthalein Indicator Reaction Equation As an indicator it turns pink to red in alkaline and is colourless in acid solutions. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is written as “phph” or hin”. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion. Phenolphthalein Indicator Reaction Equation.
From www.slideserve.com
PPT CHEMISRTY PROJECT ACIDBASE EQUILIBRIA PowerPoint Presentation Phenolphthalein Indicator Reaction Equation In this case, the weak. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. In this case, the weak acid is colourless and. Phenolphthalein Indicator Reaction Equation.
From www.youtube.com
Phenolphthalein Indicator Viva Questions pH range and Chemical Phenolphthalein Indicator Reaction Equation Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. In this case, the weak acid is colourless and its ion is bright pink. In this case, the weak. Phenolphthalein, whose structure is shown. Phenolphthalein is another commonly used indicator for titrations,. Phenolphthalein Indicator Reaction Equation.
From www.researchgate.net
Reaction of 52 with PhSH to form phenolphthalein. Download Scientific Phenolphthalein Indicator Reaction Equation In this case, the weak acid is colourless and its ion is bright pink. It has a chemical formula c 20 h 14 o 4. In this case, the weak. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. At. Phenolphthalein Indicator Reaction Equation.
From www.teachoo.com
MCQ An aqueous solution ‘A’ turns phenolphthalein solution pink. On Phenolphthalein Indicator Reaction Equation It is written as “phph” or hin”. In this case, the weak acid is colourless and its ion is bright pink. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It has a chemical formula c 20 h 14 o 4. In this case, the weak. Phenolphthalein is another commonly used. Phenolphthalein Indicator Reaction Equation.
From www.youtube.com
Phenolphthalein formation mechanism YouTube Phenolphthalein Indicator Reaction Equation In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is written as “phph” or hin”. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. Phenolphthalein is another commonly used indicator for titrations, and is another. Phenolphthalein Indicator Reaction Equation.
From www.youtube.com
Acid Base Indicator Phenolphthalein explained with experiment Phenolphthalein Indicator Reaction Equation In this case, the weak. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. Phenolphthalein, whose structure is shown. It has a chemical formula c 20 h 14 o 4. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for. Phenolphthalein Indicator Reaction Equation.
From www.vedantu.com
Explain why phenolphthalein is used as an indicator in acidbase titration. Phenolphthalein Indicator Reaction Equation Phenolphthalein, whose structure is shown. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak. In this case, the weak acid is colourless and its ion is bright pink. It is dissolved with alcohol for experiment purpose and it. Phenolphthalein Indicator Reaction Equation.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Phenolphthalein Indicator Reaction Equation Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. Phenolphthalein, whose structure is shown. As an indicator it turns pink to red in. Phenolphthalein Indicator Reaction Equation.
From www.shutterstock.com
Phenolphthalein Indicator Molecule Used Acid Base Stock Vector (Royalty Phenolphthalein Indicator Reaction Equation In this case, the weak. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. Phenolphthalein, whose structure is shown. It has a chemical formula c. Phenolphthalein Indicator Reaction Equation.
From www.toppr.com
In the mixture of (NaHCO3 + Na2CO3) volume of HCl required is x mL with Phenolphthalein Indicator Reaction Equation In this case, the weak. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is written as “phph” or hin”. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. As an. Phenolphthalein Indicator Reaction Equation.
From www.vectorstock.com
Phenolphthalein in solutions with different ph Vector Image Phenolphthalein Indicator Reaction Equation Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It has a chemical formula. Phenolphthalein Indicator Reaction Equation.
From www.chegg.com
Solved Colours of phenolphthalein indicator 100 Alkaline Phenolphthalein Indicator Reaction Equation In this case, the weak acid is colourless and its ion is bright pink. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It has a chemical formula c 20 h 14 o 4. It is written as “phph” or hin”. Phenolphthalein, whose structure is shown. Phenolphthalein is another commonly used. Phenolphthalein Indicator Reaction Equation.
From www.youtube.com
Phenols Phenolphthalein and Coupling Reaction YouTube Phenolphthalein Indicator Reaction Equation In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It has a chemical formula c 20 h 14 o 4. Phenolphthalein, whose structure is shown. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the. Phenolphthalein Indicator Reaction Equation.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Phenolphthalein Indicator Reaction Equation In this case, the weak acid is colourless and its ion is bright pink. It has a chemical formula c 20 h 14 o 4. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It. Phenolphthalein Indicator Reaction Equation.
From sciencenotes.org
Phenolphthalein Indicator Phenolphthalein Indicator Reaction Equation Phenolphthalein, whose structure is shown. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It has a chemical formula c 20 h 14 o 4. In this case, the weak. As an indicator it turns pink to red in alkaline and. Phenolphthalein Indicator Reaction Equation.
From www.chegg.com
Solved Colours of phenolphthalein indicator 100 100 cm 3 Phenolphthalein Indicator Reaction Equation It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It is written as “phph” or hin”. Phenolphthalein, whose structure is shown. In this case, the weak. It has a chemical formula c 20 h 14 o. Phenolphthalein Indicator Reaction Equation.
From www.youtube.com
Reaction of Phenol with Phthalic anhydride Phenolphthalein Organic Phenolphthalein Indicator Reaction Equation In this case, the weak acid is colourless and its ion is bright pink. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. In this case, the weak. Phenolphthalein, whose structure is shown. It is written as “phph” or hin”. It is dissolved with alcohol for experiment purpose and it is. Phenolphthalein Indicator Reaction Equation.
From www.alamy.com
Phenolphthalein indicator hires stock photography and images Alamy Phenolphthalein Indicator Reaction Equation In this case, the weak acid is colourless and its ion is bright pink. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It has a chemical formula c 20 h 14 o 4. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly. Phenolphthalein Indicator Reaction Equation.
From www.pearson.com
Phenolphthalein, a common nonprescription laxative, is also an ac Phenolphthalein Indicator Reaction Equation Phenolphthalein, whose structure is shown. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It is written as “phph” or hin”. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. It. Phenolphthalein Indicator Reaction Equation.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 Phenolphthalein Indicator Reaction Equation Phenolphthalein, whose structure is shown. In this case, the weak. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It is written as “phph” or hin”. As an indicator it turns pink to red in alkaline and is. Phenolphthalein Indicator Reaction Equation.
From www.numerade.com
SOLVED 4. Reaction of 6M NaOH with 6M HCl and phenolphthalein Phenolphthalein Indicator Reaction Equation At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It has a chemical formula c 20 h 14 o 4. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. Phenolphthalein is. Phenolphthalein Indicator Reaction Equation.
From fphoto.photoshelter.com
science chemistry titration phenolphthalein Fundamental Photographs Phenolphthalein Indicator Reaction Equation It is written as “phph” or hin”. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. Phenolphthalein, whose structure is shown. Phenolphthalein is. Phenolphthalein Indicator Reaction Equation.
From protonstalk.com
Phenolphthalein Formula, Structure, Properties, Application ProtonsTalk Phenolphthalein Indicator Reaction Equation Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. In this case, the weak acid is colourless and its ion is bright pink.. Phenolphthalein Indicator Reaction Equation.
From www.yaclass.in
Phenolphthalein as an indicator for neutralisation reaction — lesson Phenolphthalein Indicator Reaction Equation In this case, the weak. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It is written as “phph” or hin”. It is dissolved with. Phenolphthalein Indicator Reaction Equation.
From www.sciencephoto.com
Phenolphthalein Indicator Stock Image C039/1215 Science Photo Library Phenolphthalein Indicator Reaction Equation Phenolphthalein, whose structure is shown. It has a chemical formula c 20 h 14 o 4. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is written as “phph” or hin”. In this case, the weak acid is colourless and its ion is bright pink. In this case, the weak. As an indicator it turns. Phenolphthalein Indicator Reaction Equation.
From www.vedantu.com
How will you convert phenol to phenolphthalein? Phenolphthalein Indicator Reaction Equation It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. Phenolphthalein, whose structure is shown. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. In this case, the weak. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. In. Phenolphthalein Indicator Reaction Equation.
From www.numerade.com
SOLVEDDouble Displacement reactions Acid base neutralizations Add Phenolphthalein Indicator Reaction Equation Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. Phenolphthalein, whose structure is shown. It is written as “phph” or hin”. In this case, the weak. As an indicator it turns pink to red in alkaline and is. Phenolphthalein Indicator Reaction Equation.
From www.vedantu.com
Phenolphthalein indicator is used in the pH range(A) 3.2 4.4(B) 4.8 Phenolphthalein Indicator Reaction Equation At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It has a chemical formula c 20 h 14 o 4. In this case, the weak. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. It is written as “phph” or hin”. It is dissolved. Phenolphthalein Indicator Reaction Equation.
From www.nagwa.com
Question Video Identifying the Color of a Solution Containing the Acid Phenolphthalein Indicator Reaction Equation At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. In this case, the weak. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It is written as “phph” or hin”. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. Phenolphthalein,. Phenolphthalein Indicator Reaction Equation.
From www.alamy.com
Phenolphthalein indicator molecule Black and White Stock Photos Phenolphthalein Indicator Reaction Equation At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. In this case, the weak. It has a chemical formula c 20 h 14 o 4. Phenolphthalein is another commonly used indicator for titrations, and is another. Phenolphthalein Indicator Reaction Equation.
From www.priyamstudycentre.com
Phenolphthalein Indicator, Solution, Uses Phenolphthalein Indicator Reaction Equation It is dissolved with alcohol for experiment purpose and it is slightly soluble in water. In this case, the weak acid is colourless and its ion is bright pink. As an indicator it turns pink to red in alkaline and is colourless in acid solutions. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It has. Phenolphthalein Indicator Reaction Equation.
From byjus.com
in the titration of Na2CO3 by HCl using methyl orange indicator Phenolphthalein Indicator Reaction Equation At a ph of 8 or lower, the structure of phenolphthalein will be abbreviated with the formula h2p. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. It has a chemical formula c 20 h 14 o 4. It is written as “phph” or hin”. In this case, the weak. In this case, the weak acid. Phenolphthalein Indicator Reaction Equation.