Combustion Process Methane . Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. A complete combustion is a.
from www.soraesa.co
A complete combustion is a. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water.
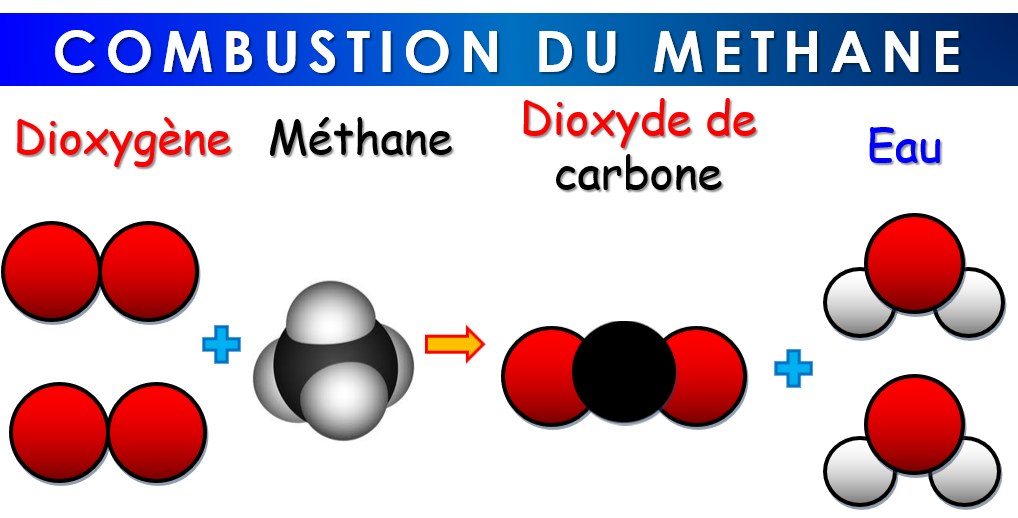
la combustion du méthane la combustion du méthane 4ème QFB66
Combustion Process Methane To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: A complete combustion is a. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely.
From www.youtube.com
Balanced Equation for the Combustion of Methane (CH4) YouTube Combustion Process Methane Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. To calculate \(\delta h^\text{0}\) for the combustion of. Combustion Process Methane.
From www.frontiersin.org
Frontiers Sequential Combustion in Steam Methane Reformers for Hydrogen and Power Production Combustion Process Methane The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. The combustion of methane is a chemical reaction where. Combustion Process Methane.
From chemicalglossary.net
Understanding the Process and Implications of Methane Combustion Reaction Chemical Glossary Combustion Process Methane Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: The combustion of methane. Combustion Process Methane.
From www.showme.com
Combustion of Methane Science ShowMe Combustion Process Methane The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. The syngas and. Combustion Process Methane.
From chemistry-europe.onlinelibrary.wiley.com
Integrated Capture and Conversion of CO2 to Methane Using a Water‐lean, CO2 Combustion Process Methane The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: A complete combustion is a. Methane is an important fuel for gas turbine and gas engine combustion, and. Combustion Process Methane.
From sciencenotes.org
Combustion Reaction Definition and Examples Combustion Process Methane To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. Stoichiometric or. Combustion Process Methane.
From www.alamy.com
The chemical equation with the reactants (methane and oxygen) and the products (carbon dioxide Combustion Process Methane Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. Methane is an. Combustion Process Methane.
From www.slideserve.com
PPT Combustion Reactions PowerPoint Presentation, free download ID4243305 Combustion Process Methane Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. To calculate \(\delta. Combustion Process Methane.
From www.britannica.com
methane Definition, Properties, Uses, & Facts Britannica Combustion Process Methane A complete combustion is a. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel. Combustion Process Methane.
From www.researchgate.net
Process flow diagram of methane synthesis from syngas produced from... Download Scientific Diagram Combustion Process Methane To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon. Combustion Process Methane.
From www.energyencyclopedia.com
Combustion of methane gas Images Free Downloads Energy Encyclopedia Combustion Process Methane Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. A complete combustion is. Combustion Process Methane.
From www.nicerweb.com
methane.html Combustion Process Methane The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. A complete combustion is a. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: The combustion of methane. Combustion Process Methane.
From www.dreamstime.com
Methane Cycle Diagram, Global Pollution Process Vector Illustration Scheme Stock Vector Combustion Process Methane Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. The syngas and methanol. Combustion Process Methane.
From www.youtube.com
Combustion methane 100pct pure O2 and 90pct YouTube Combustion Process Methane The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. To calculate \(\delta h^\text{0}\) for the combustion of one. Combustion Process Methane.
From www.tessshebaylo.com
Methane Combustion Equation Tessshebaylo Combustion Process Methane A complete combustion is a. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in. Combustion Process Methane.
From www.slideserve.com
PPT How to Use This Presentation PowerPoint Presentation, free download ID3850714 Combustion Process Methane A complete combustion is a. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: The syngas and methanol can be synthesized by partial oxidation and steam reforming. Combustion Process Methane.
From www.tessshebaylo.com
Methane Combustion Equation Complete Tessshebaylo Combustion Process Methane The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in. Combustion Process Methane.
From www.thoughtco.com
An Introduction to Combustion (Burning) Reactions Combustion Process Methane To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. The combustion. Combustion Process Methane.
From www.researchgate.net
Activation energy for methane combustion with the two catalysts. Download Scientific Diagram Combustion Process Methane Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. To calculate \(\delta h^\text{0}\) for the combustion of. Combustion Process Methane.
From www.youtube.com
Material Balances on Complete Combustion of Methane YouTube Combustion Process Methane The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows,. Combustion Process Methane.
From www.youtube.com
Combustion of Methane YouTube Combustion Process Methane To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. Methane is an. Combustion Process Methane.
From melscience.com
Interaction of methane with oxygen combustion reaction MEL Chemistry Combustion Process Methane The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. A complete combustion is a. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. The combustion of methane. Combustion Process Methane.
From methanegaskimawashi.blogspot.com
Methane Gas The Combustion Of Methane Gas Combustion Process Methane To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce. Combustion Process Methane.
From www.slideserve.com
PPT Chapter 9 Energy, Enthalpy and Thermochemistry PowerPoint Presentation ID4186375 Combustion Process Methane The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. A complete combustion is a. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The combustion of methane. Combustion Process Methane.
From dokumen.tips
(PDF) Methane Combustion in a 500 Wth ChemicalLoopingdigital.csic.es/bitstream/10261/88348/4 Combustion Process Methane A complete combustion is a. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. Methane is an important. Combustion Process Methane.
From www.slideserve.com
PPT Combustion du méthane PowerPoint Presentation, free download ID4821514 Combustion Process Methane Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce. Combustion Process Methane.
From chemicalglossary.net
Understanding the Process and Implications of Methane Combustion Reaction Chemical Glossary Combustion Process Methane To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: A complete combustion is a. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is. Combustion Process Methane.
From methanegaskimawashi.blogspot.com
Methane Gas Combustion Of Methane Gas Equation Combustion Process Methane A complete combustion is a. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as. Combustion Process Methane.
From www.tessshebaylo.com
Methane Combustion Equation Tessshebaylo Combustion Process Methane To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. A complete combustion is a. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned. Combustion Process Methane.
From www.researchgate.net
The major reaction pathways of ultralean methane combustion as... Download Scientific Diagram Combustion Process Methane The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. A complete combustion is a. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is. Combustion Process Methane.
From www.researchgate.net
SMRCLC, SteamMethane Reforming and ChemicalLooping Combustion Download Scientific Diagram Combustion Process Methane A complete combustion is a. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂),. Combustion Process Methane.
From www.youtube.com
Comment ajuster l'équation de combustion du méthane (cours 4ème) YouTube Combustion Process Methane A complete combustion is a. To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂). Combustion Process Methane.
From www.grc.nasa.gov
Combustion Combustion Process Methane The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂) and water. Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. The. Combustion Process Methane.
From www.soraesa.co
la combustion du méthane la combustion du méthane 4ème QFB66 Combustion Process Methane A complete combustion is a. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The combustion of methane is a chemical reaction where methane (ch₄) reacts with oxygen (o₂) to produce carbon dioxide (co₂), water. The syngas and methanol can be synthesized by partial oxidation and steam reforming reaction, producing consequently. The combustion of methane. Combustion Process Methane.
From www.showme.com
Balancing combustion of methane Science ShowMe Combustion Process Methane To calculate \(\delta h^\text{0}\) for the combustion of one mole of methane, first we break bonds as follows, using \(98.7 \: Methane is an important fuel for gas turbine and gas engine combustion, and the most common fuel in fundamental combustion studies. Stoichiometric or theoretical combustion is the ideal combustion process where fuel is burned completely. The combustion of methane. Combustion Process Methane.