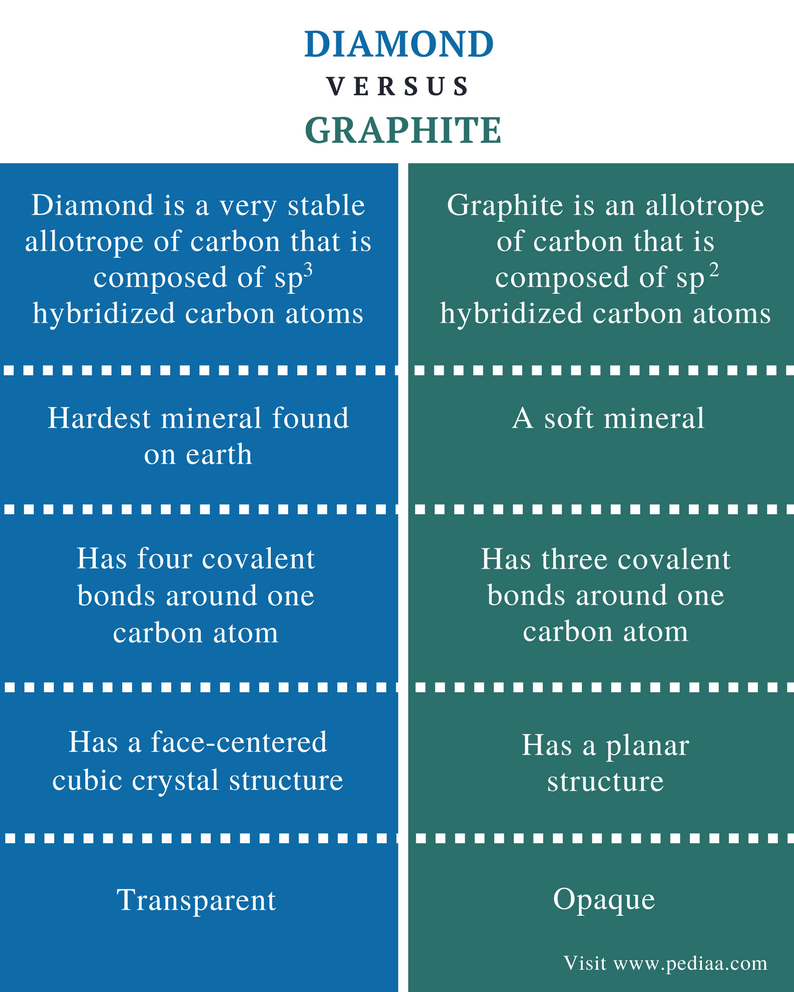
Graphite Vs Diamond Structure . On the other hand, graphite has a. These have different chemical and physical properties. The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. Graphite does conduct electricity because it. Diamond does not conduct electricity because it has no charged particles that are free to move. Diamond and graphite are allotropes of carbon. The structure of graphite consists of flat layers. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. However, the graphite’s particles join the three atoms of carbon and get associated with the plates that are parallel to each other. The main difference between diamond and graphite is that diamond is. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. In each layer the carbon atoms are arranged in a regular hexagonal array. It is soft and slippery, and its hardness is less than one on the mohs scale. We can regard each layer as a large number of benzene rings fused.
from
These have different chemical and physical properties. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. It is soft and slippery, and its hardness is less than one on the mohs scale. On the other hand, graphite has a. However, the graphite’s particles join the three atoms of carbon and get associated with the plates that are parallel to each other. We can regard each layer as a large number of benzene rings fused. Diamond does not conduct electricity because it has no charged particles that are free to move. Graphite does conduct electricity because it. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. The main difference between diamond and graphite is that diamond is.
Graphite Vs Diamond Structure The structure of graphite consists of flat layers. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. Diamond and graphite are allotropes of carbon. These have different chemical and physical properties. In each layer the carbon atoms are arranged in a regular hexagonal array. The structure of graphite consists of flat layers. However, the graphite’s particles join the three atoms of carbon and get associated with the plates that are parallel to each other. On the other hand, graphite has a. Graphite does conduct electricity because it. Diamond does not conduct electricity because it has no charged particles that are free to move. We can regard each layer as a large number of benzene rings fused. The main difference between diamond and graphite is that diamond is. The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. It is soft and slippery, and its hardness is less than one on the mohs scale.
From
Graphite Vs Diamond Structure The structure of graphite consists of flat layers. The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. In each layer the carbon atoms are arranged in a regular hexagonal array. However, the graphite’s particles join the three atoms of carbon and get associated with the plates that are parallel. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Diamond and graphite are allotropes of carbon. It is soft and slippery, and its hardness is less than one on the mohs scale.. Graphite Vs Diamond Structure.
From ar.inspiredpencil.com
Diamond Molecular Structure Graphite Vs Diamond Structure These have different chemical and physical properties. It is soft and slippery, and its hardness is less than one on the mohs scale. The structure of graphite consists of flat layers. Diamond and graphite are allotropes of carbon. We can regard each layer as a large number of benzene rings fused. Diamond and graphite have shift structures which represent their. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. These have different chemical and physical properties. We can regard each layer as a large number of benzene rings fused. The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. It is soft and. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Diamond does not conduct electricity because it has no charged particles that are free to move. Diamond and graphite are allotropes of carbon. On the other hand, graphite has a. The structure of graphite consists of flat layers. The different properties of graphite. Graphite Vs Diamond Structure.
From www.slideserve.com
PPT Atoms to Minerals PowerPoint Presentation, free download ID1383821 Graphite Vs Diamond Structure Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. The structure of graphite consists of flat layers. Graphite does conduct electricity because it. Diamond and graphite are allotropes of carbon. Diamond does not conduct electricity because it has no charged particles that are free to move. We can regard each layer as. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. However, the graphite’s particles join the three atoms of carbon and get associated with the plates that are parallel to each other. Diamond does not conduct electricity because it has no charged particles that are free to move. Graphite does conduct electricity because. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure Diamond and graphite are allotropes of carbon. We can regard each layer as a large number of benzene rings fused. However, the graphite’s particles join the three atoms of carbon and get associated with the plates that are parallel to each other. The structure of graphite consists of flat layers. Diamond does not conduct electricity because it has no charged. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure The structure of graphite consists of flat layers. Diamond and graphite are allotropes of carbon. Diamond does not conduct electricity because it has no charged particles that are free to move. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. Unlike diamond, graphite can be used as a lubricant or in pencils because. Graphite Vs Diamond Structure.
From ar.inspiredpencil.com
Graphite Structure Vs Diamond Graphite Vs Diamond Structure Diamond does not conduct electricity because it has no charged particles that are free to move. The structure of graphite consists of flat layers. We can regard each layer as a large number of benzene rings fused. These have different chemical and physical properties. Graphite does conduct electricity because it. In each layer the carbon atoms are arranged in a. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure However, the graphite’s particles join the three atoms of carbon and get associated with the plates that are parallel to each other. In each layer the carbon atoms are arranged in a regular hexagonal array. We can regard each layer as a large number of benzene rings fused. Diamond and graphite have shift structures which represent their diverse properties, and. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure The main difference between diamond and graphite is that diamond is. We can regard each layer as a large number of benzene rings fused. The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave. Graphite Vs Diamond Structure.
From ar.inspiredpencil.com
Graphite Structure Vs Diamond Graphite Vs Diamond Structure In each layer the carbon atoms are arranged in a regular hexagonal array. Diamond and graphite are allotropes of carbon. On the other hand, graphite has a. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Diamond does not conduct electricity because it has no charged particles that are free to move.. Graphite Vs Diamond Structure.
From ar.inspiredpencil.com
Diamond And Graphite Structure Graphite Vs Diamond Structure We can regard each layer as a large number of benzene rings fused. In each layer the carbon atoms are arranged in a regular hexagonal array. These have different chemical and physical properties. Diamond and graphite are allotropes of carbon. Graphite does conduct electricity because it. However, the graphite’s particles join the three atoms of carbon and get associated with. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure Diamond and graphite are allotropes of carbon. Graphite does conduct electricity because it. The main difference between diamond and graphite is that diamond is. In each layer the carbon atoms are arranged in a regular hexagonal array. These have different chemical and physical properties. It is soft and slippery, and its hardness is less than one on the mohs scale.. Graphite Vs Diamond Structure.
From differencebetweenz.com
Difference between Diamond and Graphite Difference Betweenz Graphite Vs Diamond Structure We can regard each layer as a large number of benzene rings fused. These have different chemical and physical properties. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. In each layer the carbon atoms are arranged in a regular hexagonal array. On the other hand, graphite has a. Diamond and graphite. Graphite Vs Diamond Structure.
From www.vrogue.co
Illustration Of Chemistry The Diamond Structure Each vrogue.co Graphite Vs Diamond Structure Diamond does not conduct electricity because it has no charged particles that are free to move. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. In each layer the carbon atoms are arranged in a regular hexagonal array. These have different chemical and physical properties. The structure of graphite consists of flat layers.. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. These have different chemical and physical properties.. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure Graphite does conduct electricity because it. However, the graphite’s particles join the three atoms of carbon and get associated with the plates that are parallel to each other. It is soft and slippery, and its hardness is less than one on the mohs scale. We can regard each layer as a large number of benzene rings fused. Diamond and graphite. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. Diamond and graphite are allotropes of carbon. Diamond does not conduct electricity because it has no charged particles that are free to move. We can regard each layer as a large number of benzene rings fused. On the other hand,. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. Diamond does not conduct electricity because it has no charged particles that are free to move. In each layer the carbon atoms are arranged in a regular hexagonal array. However, the graphite’s particles join the three atoms of carbon and. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure On the other hand, graphite has a. It is soft and slippery, and its hardness is less than one on the mohs scale. The main difference between diamond and graphite is that diamond is. Diamond and graphite are allotropes of carbon. Graphite does conduct electricity because it. The different properties of graphite and diamond are due to the different arrangements. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. In each layer the carbon atoms are arranged in a regular hexagonal array. The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. Graphite does conduct electricity because it. Diamond and graphite are allotropes. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure These have different chemical and physical properties. On the other hand, graphite has a. Graphite does conduct electricity because it. Diamond does not conduct electricity because it has no charged particles that are free to move. In each layer the carbon atoms are arranged in a regular hexagonal array. Unlike diamond, graphite can be used as a lubricant or in. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure The main difference between diamond and graphite is that diamond is. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. Diamond does not conduct electricity because it has no charged particles that are free to move. Diamond and graphite are allotropes of carbon. These have different chemical and physical properties. The structure of. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. On the other hand, graphite has a. These have different chemical and physical properties. However, the graphite’s particles join the three atoms of carbon and get associated. Graphite Vs Diamond Structure.
From www.youtube.com
Diamond vs Graphite Quick differences and comparison YouTube Graphite Vs Diamond Structure The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. However, the graphite’s particles join the three. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure In each layer the carbon atoms are arranged in a regular hexagonal array. These have different chemical and physical properties. On the other hand, graphite has a. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. Diamond does not conduct electricity because it has no charged particles that are free to move. Unlike. Graphite Vs Diamond Structure.
From ar.inspiredpencil.com
Graphite Structure Vs Diamond Graphite Vs Diamond Structure These have different chemical and physical properties. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. However, the graphite’s particles join the three atoms of carbon and get associated with the plates that are parallel to each other. Diamond does not conduct electricity because it has no charged particles that are free to. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure The main difference between diamond and graphite is that diamond is. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. However, the graphite’s particles join the three atoms of carbon and get associated with the plates that are parallel to each other. Diamond and graphite are allotropes of carbon. It is soft and. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure The structure of graphite consists of flat layers. On the other hand, graphite has a. We can regard each layer as a large number of benzene rings fused. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Diamond and graphite have shift structures which represent their diverse properties, and both are pure. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure The different properties of graphite and diamond are due to the different arrangements of carbon atoms in their crystal structures. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. On the other hand, graphite has a.. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure On the other hand, graphite has a. Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. It is soft and slippery, and its hardness is less than one on the mohs scale. The main difference between diamond and graphite is that diamond is. However, the graphite’s particles join the three atoms of carbon. Graphite Vs Diamond Structure.
From ar.inspiredpencil.com
Carbon Structure Of Diamond And Graphite Graphite Vs Diamond Structure Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. We can regard each layer as a large number of benzene rings fused. However, the graphite’s particles join the three atoms of carbon and get associated with the plates that are parallel to each other. In each layer the carbon atoms are arranged in. Graphite Vs Diamond Structure.
From
Graphite Vs Diamond Structure Diamond and graphite have shift structures which represent their diverse properties, and both are pure carbon. Unlike diamond, graphite can be used as a lubricant or in pencils because the layers cleave readily. In each layer the carbon atoms are arranged in a regular hexagonal array. The structure of graphite consists of flat layers. Graphite does conduct electricity because it.. Graphite Vs Diamond Structure.