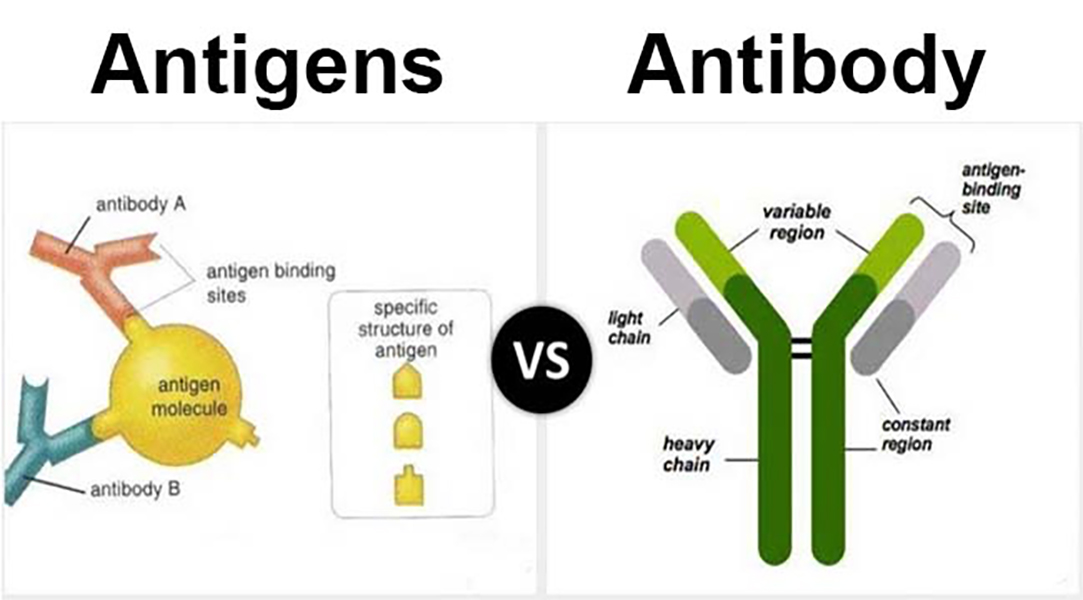
Antibody Written Description . Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted. Consequently, patents covering antibodies are among the most valuable in the patent system. Legal framework for the written description and enablement requirements. But antibody patents are being struck down left. Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. In general, antibodies can be defined by: In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. Part ii examines the evolution of the written. (1) reference to the target antigen; (3) target antigen and further antibody functional.
from biotech.gsu.edu
Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted. In general, antibodies can be defined by: In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. (1) reference to the target antigen; Part ii examines the evolution of the written. But antibody patents are being struck down left. (3) target antigen and further antibody functional. For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. Legal framework for the written description and enablement requirements.
houghton biology site
Antibody Written Description (3) target antigen and further antibody functional. Legal framework for the written description and enablement requirements. (3) target antigen and further antibody functional. Part ii examines the evolution of the written. In general, antibodies can be defined by: But antibody patents are being struck down left. Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. Consequently, patents covering antibodies are among the most valuable in the patent system. (1) reference to the target antigen; In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted.
From sciencenotes.org
Types of Antibodies and Their Functions Antibody Written Description For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. Consequently, patents covering antibodies are among the most valuable in the patent system. Legal framework for the written description and enablement requirements. Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted. Antibodies and. Antibody Written Description.
From step1.medbullets.com
Antibodies Immunology Medbullets Step 1 Antibody Written Description Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. Part ii examines the evolution of the written. In general, antibodies can be. Antibody Written Description.
From www.britannica.com
Antibody Definition, Structure, Function, & Types Britannica Antibody Written Description In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. (1) reference to the target antigen; In general, antibodies can be defined by: For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. (3) target antigen and further antibody functional. But. Antibody Written Description.
From studylib.net
Written Description Antibodies Antibody Written Description For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. (1) reference to the target antigen; Part ii examines the evolution of the written. Consequently, patents covering antibodies are among the most valuable in the patent system. But antibody patents are being struck down left. Legal framework for the. Antibody Written Description.
From biologydictionary.net
Antibody Definition, Structure and Uses Biology Dictionary Antibody Written Description In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. Part ii examines the evolution of the written. In general, antibodies can be defined by: For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. But antibody patents are being struck. Antibody Written Description.
From www.onlinebiologynotes.com
Antibody Structure, classes and functions Online Biology Notes Antibody Written Description (3) target antigen and further antibody functional. Consequently, patents covering antibodies are among the most valuable in the patent system. But antibody patents are being struck down left. In general, antibodies can be defined by: Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted. In terms of written description, claims reciting antibodies are to. Antibody Written Description.
From www.slideserve.com
PPT Written Description Antibodies Celsa TC 1600 QAS Antibody Written Description In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. Legal framework for the written description and enablement requirements. (1) reference to the target antigen; In general, antibodies can be defined by: Consequently, patents covering antibodies are among the most valuable in the patent system. For example, disclosure of an antigen fully. Antibody Written Description.
From www.onlinebiologynotes.com
Antibody Structure, classes and functions Online Biology Notes Antibody Written Description Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. (3) target antigen and further antibody functional. Part ii examines the evolution of the written. Consequently, patents covering antibodies are among the most valuable in the patent system. Antibody decisions and their compliance with. Antibody Written Description.
From www.rapidnovor.com
Primary and Secondary Antibodies in Immunoassays Antibody Written Description In general, antibodies can be defined by: Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted. Part ii examines the evolution of the written. In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. But antibody patents are being struck down left. Antibodies and statlls of. Antibody Written Description.
From www.slideserve.com
PPT Written Description Antibodies Celsa TC 1600 QAS Antibody Written Description (1) reference to the target antigen; In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. But antibody patents are being struck down left. Consequently, patents covering antibodies are among the most valuable in the patent system. Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted.. Antibody Written Description.
From www.mdpi.com
Antibodies Free FullText Understanding and Modulating Antibody Antibody Written Description For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. But antibody patents are being struck down left. Legal framework for the written description and enablement requirements. Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding. Antibody Written Description.
From www.britannica.com
Antibody Definition, Structure, Function, & Types Britannica Antibody Written Description For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. Consequently, patents covering antibodies are among the most valuable in the patent system. But antibody patents are being struck down left. Legal framework for the written description and enablement requirements. Antibody decisions and their compliance with the written description. Antibody Written Description.
From www.slideserve.com
PPT Antibody Structure and Function PowerPoint Presentation, free Antibody Written Description For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. But antibody patents are being struck down left. Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. (3) target antigen and further. Antibody Written Description.
From www.straighthealthcare.com
Antibody illustration Antibody Written Description Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. But antibody patents are being struck down left. (1) reference to the target antigen; Part ii examines the evolution of the written. Legal framework for the written description and enablement requirements. For example, disclosure. Antibody Written Description.
From www.slideserve.com
PPT Written Description Antibodies Celsa TC 1600 QAS Antibody Written Description Legal framework for the written description and enablement requirements. In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. Part ii examines the evolution of the written. Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted. Consequently, patents covering antibodies are among the most valuable in. Antibody Written Description.
From sciencevivid.com
Antibody Definition, Features, Structure, Types, Functions Sciencevivid Antibody Written Description (3) target antigen and further antibody functional. But antibody patents are being struck down left. In general, antibodies can be defined by: Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted. (1) reference to the target antigen; Consequently, patents covering antibodies are among the most valuable in the patent system. Antibodies and statlls of. Antibody Written Description.
From www.sliderbase.com
Immune System Presentation Biology Antibody Written Description (1) reference to the target antigen; For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. But antibody patents are being struck down left. In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. Antibody decisions and their compliance with the. Antibody Written Description.
From e-health.mine.nu
The Structure of an Antibody Antibody Written Description Legal framework for the written description and enablement requirements. (3) target antigen and further antibody functional. Part ii examines the evolution of the written. In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of. Antibody Written Description.
From sciencevivid.com
Antibody Definition, Features, Structure, Types, Functions Sciencevivid Antibody Written Description Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. (1) reference to the target antigen; For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. In terms of written description, claims reciting. Antibody Written Description.
From www.toppr.com
Antibody and Antigen Definitions, Importance with Questions and Videos Antibody Written Description Consequently, patents covering antibodies are among the most valuable in the patent system. (1) reference to the target antigen; In general, antibodies can be defined by: Part ii examines the evolution of the written. For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. Antibody decisions and their compliance. Antibody Written Description.
From byjus.com
Antibody Structure, Types And Functions, Antibody Written Description Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted. Legal framework for the written description and enablement requirements. (1) reference to the target antigen; Consequently, patents covering antibodies are among the most valuable in the patent system. In terms of written description, claims reciting antibodies are to be treated like claims reciting any other. Antibody Written Description.
From www.vrogue.co
Antibody Definition Functions Classification Immunolo vrogue.co Antibody Written Description For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. But antibody patents are being struck down left. Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. Legal framework for the written. Antibody Written Description.
From www.geeksforgeeks.org
Antibody Structure, Function, Types, and Production Antibody Written Description In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. Legal framework for the written description and enablement requirements. Consequently, patents covering antibodies are among the most valuable in the patent system. Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto. Antibody Written Description.
From www.slideserve.com
PPT Antibody Structure PowerPoint Presentation ID2371855 Antibody Written Description But antibody patents are being struck down left. Legal framework for the written description and enablement requirements. In general, antibodies can be defined by: For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. In terms of written description, claims reciting antibodies are to be treated like claims reciting. Antibody Written Description.
From www.slideserve.com
PPT Written Description Antibodies Celsa TC 1600 QAS Antibody Written Description Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. In general, antibodies can be defined by: (1) reference to the target antigen; But antibody patents are being struck down left. Antibody decisions and their compliance with the written description requirement powerpoint of the. Antibody Written Description.
From microbiologynotes.org
Antibody or Immunoglobulin Microbiology Notes Antibody Written Description In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. (1) reference to the target antigen; Consequently, patents covering antibodies are among the most valuable in the patent system. For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. But antibody. Antibody Written Description.
From mungfali.com
Antibody Diagram Antibody Written Description Part ii examines the evolution of the written. (1) reference to the target antigen; In general, antibodies can be defined by: Legal framework for the written description and enablement requirements. Consequently, patents covering antibodies are among the most valuable in the patent system. But antibody patents are being struck down left. Antibody decisions and their compliance with the written description. Antibody Written Description.
From study.com
Antibodies Definition, Functions, Types & Examples Lesson Antibody Written Description (1) reference to the target antigen; Consequently, patents covering antibodies are among the most valuable in the patent system. In general, antibodies can be defined by: Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. Part ii examines the evolution of the written.. Antibody Written Description.
From biotech.gsu.edu
houghton biology site Antibody Written Description (3) target antigen and further antibody functional. For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. In general, antibodies can be defined by: Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted. But antibody patents are being struck down left. In terms. Antibody Written Description.
From www.slideserve.com
PPT ANTIBODY PowerPoint Presentation, free download ID9575696 Antibody Written Description Legal framework for the written description and enablement requirements. (1) reference to the target antigen; In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. In general, antibodies can be defined by: But antibody patents are being struck down left. Antibodies and statlls of 2008 training materials the purpose of this memorandum. Antibody Written Description.
From sciencevivid.com
Antibody Definition, Features, Structure, Types, Functions Sciencevivid Antibody Written Description Consequently, patents covering antibodies are among the most valuable in the patent system. In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted. Legal framework for the written description and enablement requirements. Part ii examines the evolution. Antibody Written Description.
From pediaa.com
Difference Between Immunoglobulin and Antibody Definition, Structure Antibody Written Description In general, antibodies can be defined by: Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted. (1) reference to the target antigen; Legal framework for the written description and enablement requirements. For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. (3) target. Antibody Written Description.
From bio.libretexts.org
42.3 Antibodies Biology LibreTexts Antibody Written Description Consequently, patents covering antibodies are among the most valuable in the patent system. But antibody patents are being struck down left. In general, antibodies can be defined by: Part ii examines the evolution of the written. For example, disclosure of an antigen fully characterized by its structure, formula, chemical name, physical properties, or deposit in a public. Antibodies and statlls. Antibody Written Description.
From www.qeios.com
Antibody Definition (v1) by National Human Genome Research Institute Antibody Written Description Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. Legal framework for the written description and enablement requirements. In terms of written description, claims reciting antibodies are to be treated like claims reciting any other molecule. But antibody patents are being struck down. Antibody Written Description.
From www.mdpi.com
Antibodies Free FullText Antibody Drug Conjugates as Cancer Antibody Written Description Antibodies and statlls of 2008 training materials the purpose of this memorandum is to clarify the applicability of us pto guidance regarding the written description requirement. Consequently, patents covering antibodies are among the most valuable in the patent system. (1) reference to the target antigen; Antibody decisions and their compliance with the written description requirement powerpoint of the cbt [posted.. Antibody Written Description.