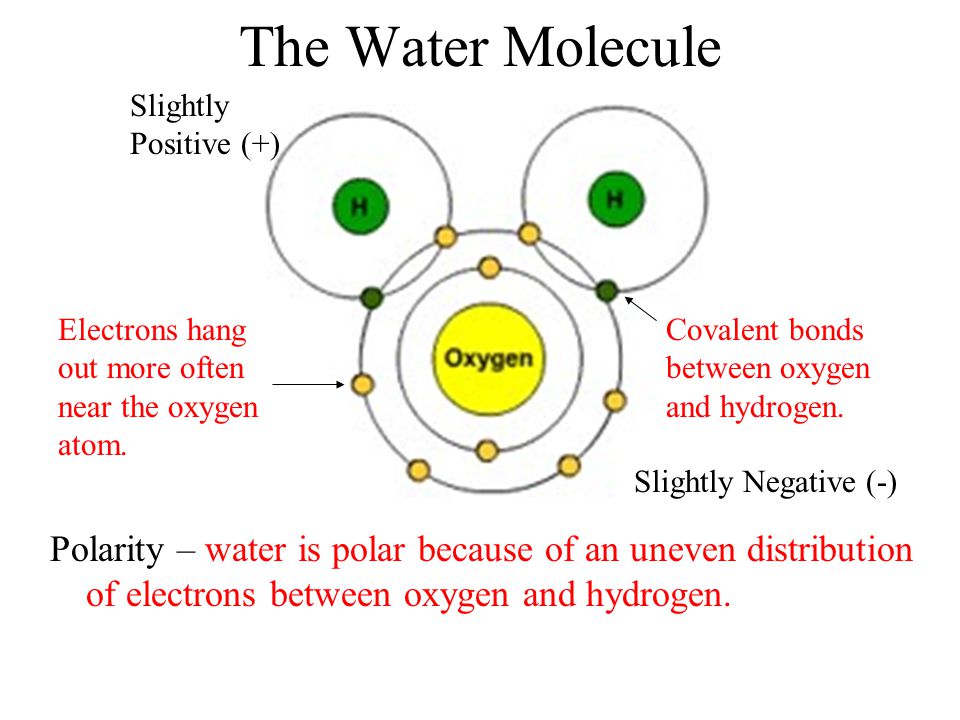
Biomolecules Water Structure . ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. Interactions with water are a major driving force for biomolecular structure and function in living systems. Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. Water has many essential roles in living organisms due to its properties: Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. Biomolecules have a wide range of sizes and structures and perform a vast. Biomolecule, any of numerous substances that are produced by cells and living organisms. The polarity of water molecules; The presence and number of.
from biostudizz.weebly.com
The presence and number of. The polarity of water molecules; Interactions with water are a major driving force for biomolecular structure and function in living systems. Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. Biomolecule, any of numerous substances that are produced by cells and living organisms. ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Biomolecules have a wide range of sizes and structures and perform a vast. Water has many essential roles in living organisms due to its properties:
WATER CHEMISTRY to Bio Stud...
Biomolecules Water Structure Biomolecules have a wide range of sizes and structures and perform a vast. In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Interactions with water are a major driving force for biomolecular structure and function in living systems. Water has many essential roles in living organisms due to its properties: The presence and number of. Biomolecule, any of numerous substances that are produced by cells and living organisms. Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Biomolecules have a wide range of sizes and structures and perform a vast. The polarity of water molecules;
From universe-review.ca
Molecules Biomolecules Water Structure Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Interactions with water are a major driving force for biomolecular structure and function in living systems. Biomolecule, any of numerous substances that are. Biomolecules Water Structure.
From quizlet.com
biology biomolecules (updated) Diagram Quizlet Biomolecules Water Structure The presence and number of. Biomolecule, any of numerous substances that are produced by cells and living organisms. Water has many essential roles in living organisms due to its properties: Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Biomolecules have a wide range of sizes and structures and perform a vast.. Biomolecules Water Structure.
From www.biologyonline.com
Micromolecule Definition and Examples Biology Online Dictionary Biomolecules Water Structure Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. Biomolecules have a wide range of sizes and structures and perform a vast. Interactions with water are a major driving force for biomolecular structure and function. Biomolecules Water Structure.
From www.slideserve.com
PPT CH 3 Biological Molecules (Biomolecules) PowerPoint Presentation Biomolecules Water Structure Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Interactions with water are a major driving force for biomolecular structure and function in living systems. Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. Each molecule of water consists of one atom of oxygen. Biomolecules Water Structure.
From biostudizz.weebly.com
WATER CHEMISTRY to Bio Stud... Biomolecules Water Structure In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Biomolecules have a wide range of sizes and structures and perform a vast. Biomolecule, any of numerous substances that are produced by cells and living organisms. ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule. Biomolecules Water Structure.
From www.studocu.com
Biomolecules (Water) 1 BiomoleculesWater The Molecule That Underpins Biomolecules Water Structure Interactions with water are a major driving force for biomolecular structure and function in living systems. Biomolecules have a wide range of sizes and structures and perform a vast. In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Biomolecule, any of numerous substances that are produced by cells and living organisms. Each. Biomolecules Water Structure.
From www.youtube.com
Biomolecules, Water, and functional groups YouTube Biomolecules Water Structure Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. Biomolecule, any of numerous substances that are produced by cells and living organisms. Interactions with water are a major driving force for biomolecular structure and function in living systems. ‘water as a biomolecule’ considers how water influences biological processes and. Biomolecules Water Structure.
From notesbard.com
Biomolecules Definition, Types, Structure, & Examples Biomolecules Water Structure The polarity of water molecules; In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. The presence and number of. Biomolecule, any of numerous substances that are produced by cells and living organisms. Biomolecules. Biomolecules Water Structure.
From www.majordifferences.com
Four Biomolecules Structure and Function Comparison Chart Biomolecules Water Structure Biomolecule, any of numerous substances that are produced by cells and living organisms. In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Water has many essential roles in living organisms due to its properties: Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. The. Biomolecules Water Structure.
From proper-cooking.info
Nucleic Acids Structures Properties And Functions Biomolecules Water Structure ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. The presence and number of. The polarity of water molecules; Biomolecules have a wide range of sizes and structures and perform a vast. Interactions. Biomolecules Water Structure.
From www.sciencelearn.org.nz
Biomolecules — Science Learning Hub Biomolecules Water Structure Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. Interactions with water are a major driving force for biomolecular structure and function in living systems. ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. Biomolecule, any of numerous substances. Biomolecules Water Structure.
From www.thesciencehive.co.uk
Biological Molecules (A Level) — the science hive Biomolecules Water Structure Interactions with water are a major driving force for biomolecular structure and function in living systems. Water has many essential roles in living organisms due to its properties: In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Biomolecule, any of numerous substances that are produced by cells and living organisms. Biomolecules have. Biomolecules Water Structure.
From www.slideshare.net
Protein as a Biomolecule Biomolecules Water Structure Biomolecule, any of numerous substances that are produced by cells and living organisms. Interactions with water are a major driving force for biomolecular structure and function in living systems. The presence and number of. The polarity of water molecules; In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Water has many essential. Biomolecules Water Structure.
From www.slideserve.com
PPT CH 3 Biological Molecules (Biomolecules) PowerPoint Presentation Biomolecules Water Structure Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. The polarity of water molecules; ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. Biomolecule, any of numerous substances that are produced by cells and living organisms. The presence and. Biomolecules Water Structure.
From www.bol.com
Structure and Dynamics of Water around Biomolecules 9786202529051 Biomolecules Water Structure Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. Biomolecule, any of numerous substances that are produced by cells and living organisms. The presence and number of. Biomolecules have a wide range of. Biomolecules Water Structure.
From www.exobiologie.fr
The role of water in the structure and function of biological Biomolecules Water Structure In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Interactions with water are a major driving force for biomolecular structure and function in living systems. Each molecule of water consists of one atom of oxygen. Biomolecules Water Structure.
From www.controlled-molecule-imaging.org
How biomolecules react to UV light Biomolecules Water Structure Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Biomolecules have a wide range of sizes and structures and perform a vast. The presence and number of. In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. ‘water as a biomolecule’ considers how water influences. Biomolecules Water Structure.
From www.researchgate.net
Schematic diagram showing interaction of water molecules with Biomolecules Water Structure The polarity of water molecules; Biomolecules have a wide range of sizes and structures and perform a vast. Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. The presence and number of. ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. Water. Biomolecules Water Structure.
From biologycarol.teachable.com
Biomolecules biologycarol Biomolecules Water Structure Interactions with water are a major driving force for biomolecular structure and function in living systems. In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Biomolecules have a wide range of sizes and structures and perform a vast. ‘water as a biomolecule’ considers how water influences biological processes and what properties of. Biomolecules Water Structure.
From www.studocu.com
Biomolecules (Water) 3 BiomoleculesWater Bases and Acids Anything Biomolecules Water Structure ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. Biomolecules have a wide range of sizes and structures and perform a vast. In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. The presence and number of. The polarity of water molecules; Water. Biomolecules Water Structure.
From www.slideshare.net
Biomolecules and water Biomolecules Water Structure Biomolecules have a wide range of sizes and structures and perform a vast. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. Interactions with water are a major driving force for biomolecular structure and function in living systems. The presence and number of. In water, the nonbonding electrons are. Biomolecules Water Structure.
From www.tes.com
Biomolecules GCSE Teaching Resources Biomolecules Water Structure Water has many essential roles in living organisms due to its properties: The polarity of water molecules; Biomolecule, any of numerous substances that are produced by cells and living organisms. Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. ‘water as a biomolecule’ considers how water influences biological processes and what properties. Biomolecules Water Structure.
From www.slideserve.com
PPT Biomolecules in Water PowerPoint Presentation, free download ID Biomolecules Water Structure In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Interactions with water are a major driving force for biomolecular structure and function in living systems. Each molecule of water consists of one atom of oxygen. Biomolecules Water Structure.
From biologiadelcadalso.blogspot.com
BIOELEMENTOS Y BIOMOLÉCULAS Biomolecules Water Structure Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. The polarity of water molecules; Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. Biomolecules. Biomolecules Water Structure.
From revision-world-alevel.blogspot.com
Biology Biological molecules Water Biomolecules Water Structure Interactions with water are a major driving force for biomolecular structure and function in living systems. Water has many essential roles in living organisms due to its properties: Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. Biomolecule, any of numerous substances that are produced by cells and living organisms. The presence. Biomolecules Water Structure.
From quizlet.com
Biomolecules (aka Macromolecules) Diagram Quizlet Biomolecules Water Structure Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. Biomolecules have a wide range of sizes and structures and perform a vast. The polarity of water molecules; In water, the nonbonding electrons. Biomolecules Water Structure.
From www.vecteezy.com
The Water Molecule 12025558 PNG Biomolecules Water Structure Water has many essential roles in living organisms due to its properties: In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Interactions with water are a major driving force for biomolecular structure and function in. Biomolecules Water Structure.
From www.researchgate.net
2. Illustration comparing allatom structures of biomolecules with Biomolecules Water Structure ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Biomolecules have a wide. Biomolecules Water Structure.
From conductscience.com
Biomolecules Types and Functions Conduct Science Biomolecules Water Structure Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. Interactions with water are a major driving force for biomolecular structure and function in living systems. Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. ‘water as a biomolecule’ considers how water. Biomolecules Water Structure.
From www.researchgate.net
Schematic diagram showing interaction of water molecules with Biomolecules Water Structure Water has many essential roles in living organisms due to its properties: Biomolecules have a wide range of sizes and structures and perform a vast. Biomolecule, any of numerous substances that are produced by cells and living organisms. ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. In water, the. Biomolecules Water Structure.
From www.inspiritvr.com
Structure and Function of Biological Macromolecules Study Guide Biomolecules Water Structure Biomolecules have a wide range of sizes and structures and perform a vast. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. Interactions with water are a major driving force for biomolecular structure and function in living systems. Water is an essential participant in the stability, structure, dynamics, and. Biomolecules Water Structure.
From www.hcrowder.com
Biomolecules Biomolecules Water Structure The presence and number of. Water has many essential roles in living organisms due to its properties: Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Each molecule of water consists of one atom of oxygen and two atoms of hydrogen, so it has the chemical. The polarity of water molecules; Interactions. Biomolecules Water Structure.
From www.learninsta.com
Biomolecules of Proteins Biomolecules Water Structure Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. In water, the nonbonding electrons are the h‐bond acceptors and the hydrogen atoms are the h‐bond donors. Interactions with water are a major driving force for biomolecular structure and function in living systems. Each molecule of water consists of one atom of oxygen. Biomolecules Water Structure.
From www.youtube.com
4 Biological Molecules Structure and Their Function A quick guide Biomolecules Water Structure Water is an essential participant in the stability, structure, dynamics, and function of proteins and other biomolecules. Biomolecule, any of numerous substances that are produced by cells and living organisms. ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. Biomolecules have a wide range of sizes and structures and perform. Biomolecules Water Structure.
From learn.careers360.com
What is Biomolecules Definition of Biomolecules, Notes, Examples, Books Biomolecules Water Structure Numerous studies have shown that water structure and dynamics are altered in the close vicinity of biomolecules. Interactions with water are a major driving force for biomolecular structure and function in living systems. ‘water as a biomolecule’ considers how water influences biological processes and what properties of the water molecule enable it. Biomolecule, any of numerous substances that are produced. Biomolecules Water Structure.