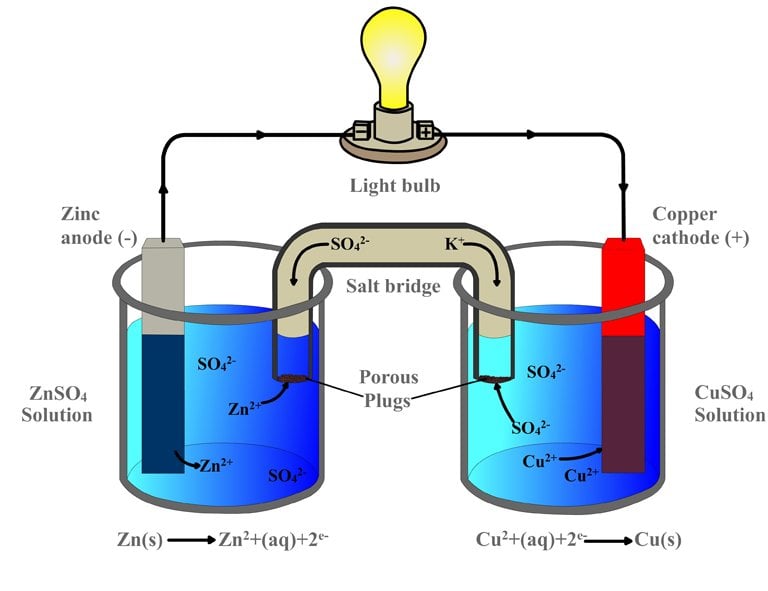
Zinc And Copper Electrolytes . The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal.
from www.scienceabc.com
A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal.
Galvanic Cell Definition, Diagram And Working
Zinc And Copper Electrolytes A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper.
From www.alamy.com
Voltage Column with plates of zinc and copper and a plate of Zinc And Copper Electrolytes A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. The circuit is a loop. Zinc And Copper Electrolytes.
From www.comsol.fr
5 W’s for 200 Years of COMSOL Blog Zinc And Copper Electrolytes What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. A simple electrochemical cell can. Zinc And Copper Electrolytes.
From www.alamy.com
Voltage Column with plates of zinc and copper and a plate of Zinc And Copper Electrolytes A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A typical cell might consist of two pieces of metal, one zinc. Zinc And Copper Electrolytes.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Zinc And Copper Electrolytes The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. A typical cell might consist of two pieces of metal, one zinc and the other. Zinc And Copper Electrolytes.
From www.slideserve.com
PPT Electrodeposition PowerPoint Presentation, free download ID923862 Zinc And Copper Electrolytes A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. The circuit is a loop. Zinc And Copper Electrolytes.
From www.askiitians.com
Applications Of Electrolysis Study Material for IIT JEE askIITians Zinc And Copper Electrolytes The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A typical cell might consist of two pieces of metal, one zinc and the other copper,. Zinc And Copper Electrolytes.
From www.learnatnoon.com
How are metals refined by the electrolytic process? Describe the Zinc And Copper Electrolytes A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per. Zinc And Copper Electrolytes.
From rohanfersmorrison.blogspot.com
Identify the Conditions for a Standard Electrochemical Cell. Zinc And Copper Electrolytes A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed. Zinc And Copper Electrolytes.
From www.semanticscholar.org
Figure 1 from Coupling of Zinc Porphyrin Dyes and Copper Electrolytes Zinc And Copper Electrolytes A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. What induces electrons to leave the atoms around the part of. Zinc And Copper Electrolytes.
From www.askiitians.com
Daniell Cell Study Material for IIT JEE askIITians Zinc And Copper Electrolytes A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A simple electrochemical cell can. Zinc And Copper Electrolytes.
From www.science.org
Zinccopper dualion electrolytes to suppress dendritic growth and Zinc And Copper Electrolytes The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. A typical cell might consist of two pieces of metal, one zinc and the other. Zinc And Copper Electrolytes.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Zinc And Copper Electrolytes The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and. Zinc And Copper Electrolytes.
From www.semanticscholar.org
Figure 1 from Coupling of Zinc Porphyrin Dyes and Copper Electrolytes Zinc And Copper Electrolytes What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the. Zinc And Copper Electrolytes.
From fphoto.photoshelter.com
science chemistry redox reaction electrochemical cell Fundamental Zinc And Copper Electrolytes A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. The circuit is a loop through the zinc, the wire, the copper,. Zinc And Copper Electrolytes.
From colouremployer8.gitlab.io
Marvelous Copper Zinc Battery Reaction Cie Chemistry A Level Syllabus Zinc And Copper Electrolytes What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. The circuit is a loop through the zinc, the wire, the copper,. Zinc And Copper Electrolytes.
From chemwiki.ucdavis.edu
Voltaic Cells Chemwiki Zinc And Copper Electrolytes Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. What induces electrons to. Zinc And Copper Electrolytes.
From www.researchgate.net
Cyclic voltammogram of electrolyte containing copper chloride, zinc Zinc And Copper Electrolytes A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The circuit is a loop through the. Zinc And Copper Electrolytes.
From www.science.org
Zinccopper dualion electrolytes to suppress dendritic growth and Zinc And Copper Electrolytes A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. Zinc dissolves in lemon juice, leaving zinc. Zinc And Copper Electrolytes.
From 2012books.lardbucket.org
Electrochemistry Zinc And Copper Electrolytes What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. The circuit is a loop through the zinc, the. Zinc And Copper Electrolytes.
From www.simplechemconcepts.com
Simple Cells in Electrolysis topic Zinc And Copper Electrolytes A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed. Zinc And Copper Electrolytes.
From www.science.org
Zinccopper dualion electrolytes to suppress dendritic growth and Zinc And Copper Electrolytes Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. The circuit is a loop through the zinc, the wire, the. Zinc And Copper Electrolytes.
From www.alamy.com
lowcost experiment, voltaic cell with a copper strip and zinc strip Zinc And Copper Electrolytes A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. The circuit is a loop. Zinc And Copper Electrolytes.
From electricala2z.com
Voltaic Cell Construction Working Examples Zinc And Copper Electrolytes The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and. Zinc And Copper Electrolytes.
From www.alamy.com
Electroplating with copper using copper sulfate electrolyte Zinc And Copper Electrolytes A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed. Zinc And Copper Electrolytes.
From classnotes.org.in
Extraction of Copper and Zinc Chemistry, Class 12, General Principles Zinc And Copper Electrolytes A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. A simple electrochemical cell. Zinc And Copper Electrolytes.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Zinc And Copper Electrolytes What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. The circuit is a loop through the zinc, the. Zinc And Copper Electrolytes.
From www.science.org
Zinccopper dualion electrolytes to suppress dendritic growth and Zinc And Copper Electrolytes The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed. Zinc And Copper Electrolytes.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Zinc And Copper Electrolytes What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the. Zinc And Copper Electrolytes.
From courses.lumenlearning.com
Electrolysis Chemistry Zinc And Copper Electrolytes A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. The circuit is a. Zinc And Copper Electrolytes.
From saylordotorg.github.io
Standard Potentials Zinc And Copper Electrolytes A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. A simple electrochemical cell can be produced by dipping two different. Zinc And Copper Electrolytes.
From fphoto.photoshelter.com
science chemistry redox reaction electrochemical cell Fundamental Zinc And Copper Electrolytes The circuit is a loop through the zinc, the wire, the copper, and the electrolyte, back to the zinc. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. A typical cell might consist of two pieces of metal, one zinc and the other. Zinc And Copper Electrolytes.
From www.mdpi.com
Metals Free FullText Progress on Electrodeposition of Metals and Zinc And Copper Electrolytes A simple electrochemical cell can be produced by dipping two different metals into an electrolyte and connecting them via wires and a voltmeter,. Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. What induces electrons to leave the atoms around the part of. Zinc And Copper Electrolytes.
From eplating.co.uk
Zinc Plating Electrolyte ePlating Zinc And Copper Electrolytes Zinc dissolves in lemon juice, leaving zinc ions (zn 2+) in the juice, while the two electrons per atom move through the wire toward the copper. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. The circuit is a loop through the zinc, the. Zinc And Copper Electrolytes.
From pubs.sciepub.com
Figure 14. CuZn battery inside the beaker with the stirred electrolyte Zinc And Copper Electrolytes A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. A simple electrochemical cell can. Zinc And Copper Electrolytes.
From brainly.in
draw the diagram showing the electrolytic refining of copper Brainly.in Zinc And Copper Electrolytes A typical cell might consist of two pieces of metal, one zinc and the other copper, each immersed each in a solution containing a dissolved salt of the corresponding metal. What induces electrons to leave the atoms around the part of the zinc metal in a solution to travel up along that zinc metal and. Zinc dissolves in lemon juice,. Zinc And Copper Electrolytes.