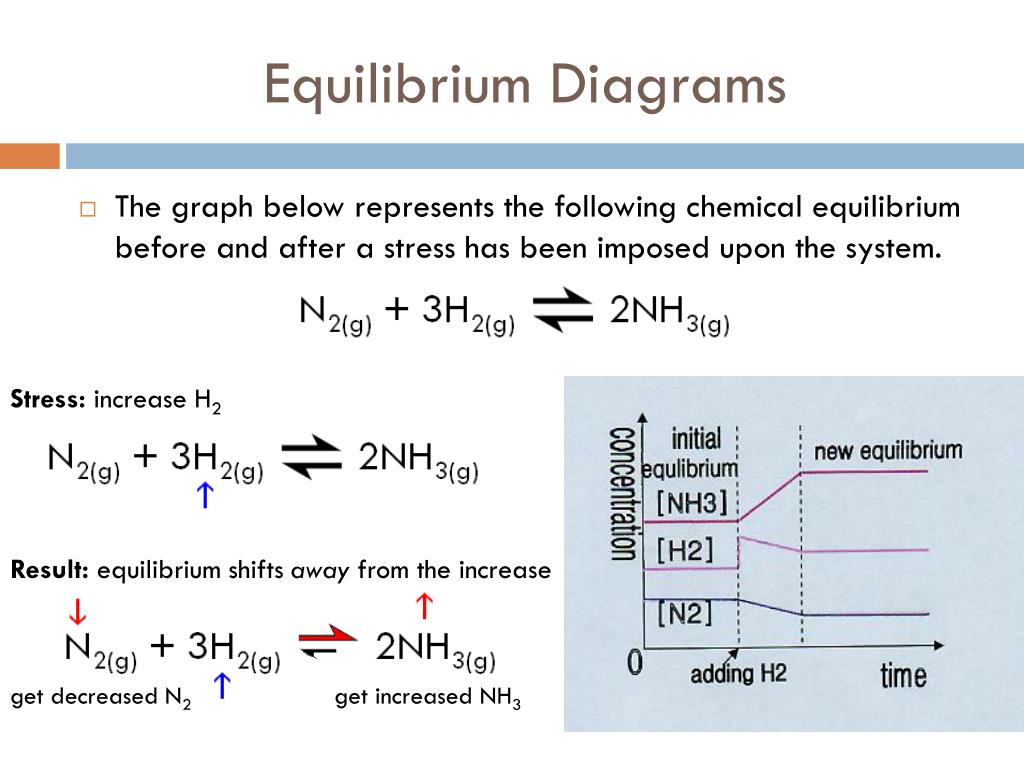
Justify Why Catalysts Do Not Shift The Equilibrium Position . A catalyst speeds up both the. Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. Hence, although the system will. Catalysts do not affect equilibrium. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is said to shift to the right when the forward reaction is favoured. When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means it becomes less negative. 3h₂ + n₂ ⇋ 2nh₃. This means that there is an increase in the amount of products formed. The position of equilibrium is. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. In such a reaction a catalyst speeds up both the forward and the backward reactions.
from www.slideserve.com
As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. Hence, although the system will. 3h₂ + n₂ ⇋ 2nh₃. This means that there is an increase in the amount of products formed. When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means it becomes less negative. In such a reaction a catalyst speeds up both the forward and the backward reactions. The position of equilibrium is said to shift to the right when the forward reaction is favoured. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. A catalyst speeds up both the. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed.
PPT Chemical Equilibrium PowerPoint Presentation, free download ID
Justify Why Catalysts Do Not Shift The Equilibrium Position As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Catalysts do not affect equilibrium. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. A catalyst speeds up both the. 3h₂ + n₂ ⇋ 2nh₃. This means that there is an increase in the amount of products formed. In such a reaction a catalyst speeds up both the forward and the backward reactions. Hence, although the system will. As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. The position of equilibrium is said to shift to the right when the forward reaction is favoured. The position of equilibrium is. When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means it becomes less negative.
From hxeaspeqb.blob.core.windows.net
Why Do Catalysts Not Affect The Position Of Equilibrium at Erica Murphy Justify Why Catalysts Do Not Shift The Equilibrium Position As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. This means that there is an increase in the amount of products formed. 3h₂ + n₂ ⇋ 2nh₃. Catalysts do not affect equilibrium. The position of equilibrium is. Hence, although the system will. The position of equilibrium is said to shift to. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From slideplayer.com
The student will learn ppt download Justify Why Catalysts Do Not Shift The Equilibrium Position When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means it becomes less negative. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is. This means that there is an increase in the amount of products formed. As we learned during our study. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.chegg.com
Solved Le Chatelier's Principle 1. Which way will the Justify Why Catalysts Do Not Shift The Equilibrium Position When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means it becomes less negative. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is said to shift to the right when the forward reaction is favoured. This means that there is an increase. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From slideplayer.com
Chapter 9 Chemical Reactions. ppt download Justify Why Catalysts Do Not Shift The Equilibrium Position A catalyst speeds up both the. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. This means that there is an increase in the amount of products formed. Adding a catalyst to this, or any. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.savemyexams.com
The Position of Equilibrium Edexcel IGCSE Chemistry Revision Notes 2019 Justify Why Catalysts Do Not Shift The Equilibrium Position As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. Catalysts do not affect equilibrium. This means that there is an increase in the amount of products formed. In such a reaction a catalyst speeds up both the forward and the backward reactions. A catalyst speeds up both the. When we increase. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Justify Why Catalysts Do Not Shift The Equilibrium Position In such a reaction a catalyst speeds up both the forward and the backward reactions. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is. Hence, although the system will. A catalyst speeds up both the. The position of equilibrium is said to shift to the right when the. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.21C Understand Why a Catalyst Does Not Affect Justify Why Catalysts Do Not Shift The Equilibrium Position Catalysts do not affect equilibrium. A catalyst speeds up both the. When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means it becomes less negative. Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. As we learned during our study of kinetics, a catalyst can. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.youtube.com
Equilibrium Graphs grade 12 Catalyst YouTube Justify Why Catalysts Do Not Shift The Equilibrium Position Hence, although the system will. In such a reaction a catalyst speeds up both the forward and the backward reactions. This means that there is an increase in the amount of products formed. When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means it becomes less negative. Adding a catalyst to this, or any. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.nagwa.com
Question Video Identifying the Change to an Equilibrium System That Justify Why Catalysts Do Not Shift The Equilibrium Position Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. 3h₂ + n₂ ⇋ 2nh₃. Hence, although the system will. The position of equilibrium is said to shift to the right when the forward reaction is favoured. The position of equilibrium is. In such a reaction a catalyst speeds up both the. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From slideplayer.com
A guide for A level students KNOCKHARDY PUBLISHING ppt download Justify Why Catalysts Do Not Shift The Equilibrium Position Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is. The position of equilibrium is said to shift to the right when the forward reaction is favoured. Catalysts do not affect equilibrium. This means that there is an increase in the amount of products formed. Hence, although the system. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Justify Why Catalysts Do Not Shift The Equilibrium Position 3h₂ + n₂ ⇋ 2nh₃. The position of equilibrium is said to shift to the right when the forward reaction is favoured. Catalysts do not affect equilibrium. Hence, although the system will. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. As we learned during our study of kinetics, a catalyst can speed. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From general.chemistrysteps.com
Le Chateliers Principle Chemistry Steps Justify Why Catalysts Do Not Shift The Equilibrium Position Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. A catalyst speeds up both the. In such a reaction a catalyst speeds up both the forward and the backward reactions. As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. When we increase the temperature,. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.chemicals.co.uk
Chemistry A Level Revision Equilibrium Justify Why Catalysts Do Not Shift The Equilibrium Position Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. Catalysts do not affect equilibrium. As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. 3h₂ + n₂. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.slideserve.com
PPT Le Châtelier’s Principle PowerPoint Presentation, free download Justify Why Catalysts Do Not Shift The Equilibrium Position The position of equilibrium is said to shift to the right when the forward reaction is favoured. As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is. This means that there. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID2170548 Justify Why Catalysts Do Not Shift The Equilibrium Position The position of equilibrium is. Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. The position of equilibrium is said to shift to the right when the forward reaction is favoured. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. 3h₂ + n₂ ⇋. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From crappychemistrynotes.wordpress.com
[Not finished] Why do catalysts not change the equillbrium positions Justify Why Catalysts Do Not Shift The Equilibrium Position Hence, although the system will. A catalyst speeds up both the. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is. Catalysts do not affect equilibrium. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. In such a reaction a catalyst speeds. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From ecampusontario.pressbooks.pub
Chemical Equilibrium First Year General Chemistry Justify Why Catalysts Do Not Shift The Equilibrium Position Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is. Catalysts do not affect equilibrium. The position of equilibrium is said to shift to the right when the forward reaction is favoured. As we learned during our study of kinetics, a catalyst can speed up the rate of a. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.numerade.com
SOLVED6 Which explanation best describes why a catalyst does not Justify Why Catalysts Do Not Shift The Equilibrium Position 3h₂ + n₂ ⇋ 2nh₃. Catalysts do not affect equilibrium. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is. When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means it becomes less negative. Le chatelier's principle addresses how an equilibrium shifts when. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From byjus.com
Why do catalyst not affect equilibrium? Justify Why Catalysts Do Not Shift The Equilibrium Position Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. In such a reaction a catalyst speeds up both the forward and the backward reactions. 3h₂ + n₂ ⇋ 2nh₃. Catalysts do not affect equilibrium. A catalyst speeds up both the. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.nagwa.com
Question Video Identifying What Is Meant by the Equilibrium Position Justify Why Catalysts Do Not Shift The Equilibrium Position 3h₂ + n₂ ⇋ 2nh₃. The position of equilibrium is. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is said to shift to the right when the forward reaction is favoured. Hence, although the system will. As we learned during our study of kinetics, a catalyst can speed. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.youtube.com
Quick review equilibrium shifts explained YouTube Justify Why Catalysts Do Not Shift The Equilibrium Position Hence, although the system will. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. In such a reaction a catalyst speeds up both the forward and the backward reactions. The position of equilibrium is. A. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From slideplayer.com
& Equilibrium ppt download Justify Why Catalysts Do Not Shift The Equilibrium Position A catalyst speeds up both the. Hence, although the system will. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Catalysts do not affect equilibrium. The position of equilibrium is. This means that there is an increase in the amount of products formed. When we increase the temperature, that exponent gets smaller in. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From h-o-m-e.org
Catalyst Tipping the Scales of Equilibrium Justify Why Catalysts Do Not Shift The Equilibrium Position As we learned during our study of kinetics, a catalyst can speed up the rate of a reaction. In such a reaction a catalyst speeds up both the forward and the backward reactions. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. This means that there is an increase in the amount of. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 Justify Why Catalysts Do Not Shift The Equilibrium Position In such a reaction a catalyst speeds up both the forward and the backward reactions. When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means it becomes less negative. Catalysts do not affect equilibrium. Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. Le chatelier's. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Justify Why Catalysts Do Not Shift The Equilibrium Position The position of equilibrium is said to shift to the right when the forward reaction is favoured. The position of equilibrium is. In such a reaction a catalyst speeds up both the forward and the backward reactions. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Hence, although the system will. Adding a. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Justify Why Catalysts Do Not Shift The Equilibrium Position 3h₂ + n₂ ⇋ 2nh₃. Hence, although the system will. Catalysts do not affect equilibrium. Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. The position of equilibrium is. The position of equilibrium is said to shift to the right when the forward reaction is favoured. This means that there is. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From slideplayer.com
Factors That Affect Equilibrium ppt download Justify Why Catalysts Do Not Shift The Equilibrium Position Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is. The position of equilibrium is said to shift to the right when the forward reaction is favoured. Hence, although the system will. Adding a catalyst to this, or any other equilibrium system, will not affect the position of an. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.slideserve.com
PPT Le Châtelier’s Principle PowerPoint Presentation, free download Justify Why Catalysts Do Not Shift The Equilibrium Position Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. The position of equilibrium is. When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means it becomes less negative. 3h₂. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID Justify Why Catalysts Do Not Shift The Equilibrium Position Catalysts do not affect equilibrium. The position of equilibrium is. A catalyst speeds up both the. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. Hence, although the system will. When we increase the temperature,. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.chemistrystudent.com
Equilibrium (ALevel) ChemistryStudent Justify Why Catalysts Do Not Shift The Equilibrium Position Catalysts do not affect equilibrium. Hence, although the system will. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.numerade.com
SOLVED Addition of a catalyst to the reaction mixture would cause the Justify Why Catalysts Do Not Shift The Equilibrium Position In such a reaction a catalyst speeds up both the forward and the backward reactions. The position of equilibrium is. When we increase the temperature, that exponent gets smaller in magnitude (closer to zero), which means it becomes less negative. Hence, although the system will. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From sciencenotes.org
Le Chatelier's Principle Justify Why Catalysts Do Not Shift The Equilibrium Position This means that there is an increase in the amount of products formed. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Catalysts do not affect equilibrium. 3h₂ + n₂ ⇋ 2nh₃. When we increase the temperature,. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.numerade.com
SOLVEDWhy do catalysts not influence the chemical equilibrium? Justify Why Catalysts Do Not Shift The Equilibrium Position This means that there is an increase in the amount of products formed. Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. The position of equilibrium is. In such a reaction a catalyst speeds up both the forward and the backward reactions. Le chatelier’s principle addresses how an equilibrium shifts when. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.numerade.com
SOLVED Question 4. Position of Equilibrium. Consider the following Justify Why Catalysts Do Not Shift The Equilibrium Position This means that there is an increase in the amount of products formed. Le chatelier’s principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. A catalyst speeds up both the. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Hence, although the system will. Catalysts do not affect. Justify Why Catalysts Do Not Shift The Equilibrium Position.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID3480898 Justify Why Catalysts Do Not Shift The Equilibrium Position Adding a catalyst to this, or any other equilibrium system, will not affect the position of an equilibrium. This means that there is an increase in the amount of products formed. Catalysts do not affect equilibrium. The position of equilibrium is. Le chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed. Hence, although the. Justify Why Catalysts Do Not Shift The Equilibrium Position.