Test Method Validation Acceptance Criteria . Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. The choice of surrogate matrix should be scientifically justified. Matrices may be acceptable for analytical method validation. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. General statement on acceptance criteria for the process. These objectives are described with.
from www.professionalqa.com
A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. General statement on acceptance criteria for the process. The choice of surrogate matrix should be scientifically justified. These objectives are described with. Matrices may be acceptable for analytical method validation. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be.
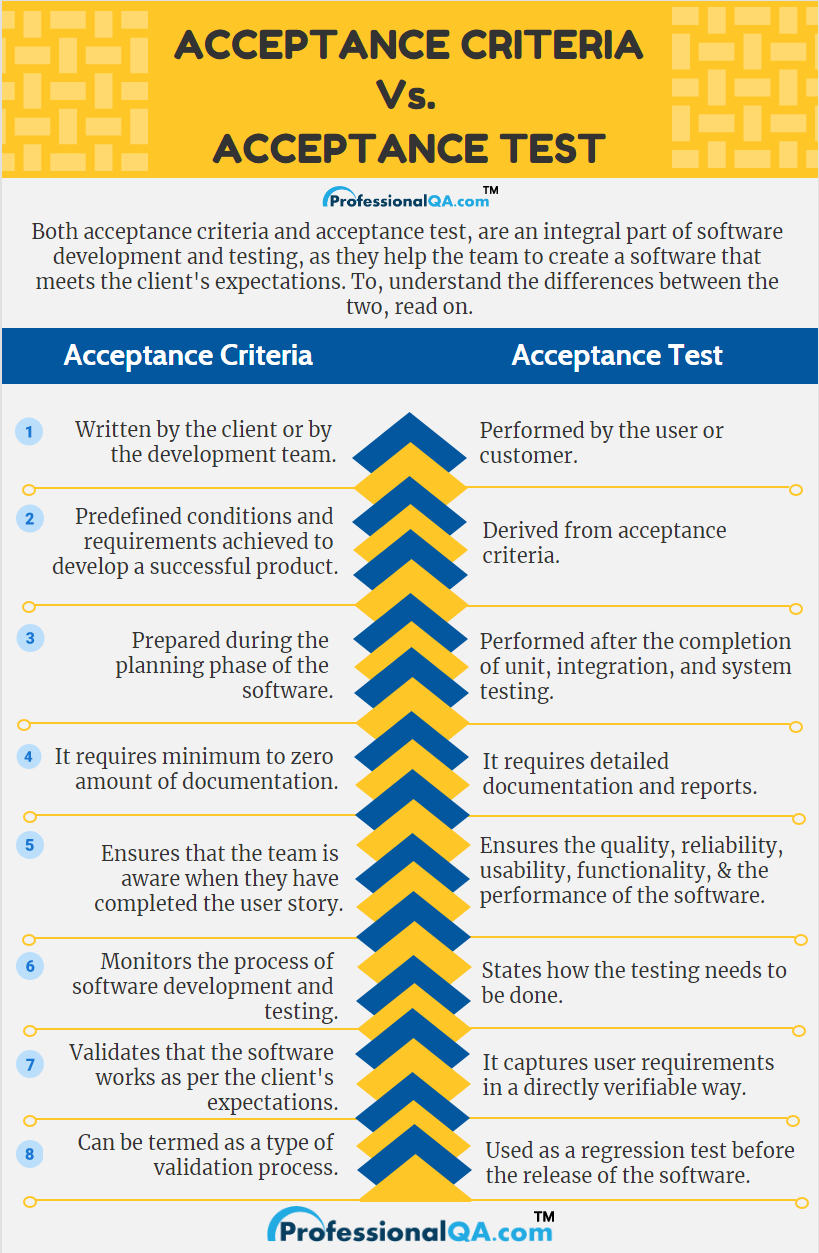
Acceptance Criteria Vs Acceptance Test
Test Method Validation Acceptance Criteria These objectives are described with. General statement on acceptance criteria for the process. These objectives are described with. The choice of surrogate matrix should be scientifically justified. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. Matrices may be acceptable for analytical method validation.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Acceptance Criteria General statement on acceptance criteria for the process. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. These objectives are described with. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. Reproduce the necessary conditions and obtain results within the proposed. Test Method Validation Acceptance Criteria.
From www.scribd.com
Validation Criteria Checklist PDF Verification And Validation Test Method Validation Acceptance Criteria The choice of surrogate matrix should be scientifically justified. General statement on acceptance criteria for the process. These objectives are described with. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. Matrices. Test Method Validation Acceptance Criteria.
From kullananlarkulubu.com
Analytical Method Validation for Quality Assurance and Process Test Method Validation Acceptance Criteria Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. General statement on acceptance criteria for the process. The choice of surrogate matrix should be scientifically justified. It provides guidance and recommendations on how to derive and evaluate the. Test Method Validation Acceptance Criteria.
From www.softwaretestinghelp.com
Validation Testing Ultimate Guide Test Method Validation Acceptance Criteria Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. The choice of surrogate matrix should be scientifically justified. General statement on acceptance criteria for the process. These objectives are described with. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. It provides guidance and recommendations on how. Test Method Validation Acceptance Criteria.
From dev.powerslides.com
Acceptance Criteria Template Agile Development Templates Test Method Validation Acceptance Criteria Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. The choice of surrogate matrix should be scientifically justified. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. These objectives are described with. A validation study is designed to provide sufficient evidence that the analytical procedure meets its. Test Method Validation Acceptance Criteria.
From www.semanticscholar.org
Table I from Method validation by phase of development An acceptable Test Method Validation Acceptance Criteria It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. General statement on acceptance criteria for the process. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. Matrices may be acceptable for analytical method validation. Verification of a test method demonstrates that the laboratory has met the test method’s. Test Method Validation Acceptance Criteria.
From www.slideserve.com
PPT Validation of Analytical Methods PowerPoint Presentation, free Test Method Validation Acceptance Criteria These objectives are described with. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. The choice of surrogate matrix should be scientifically justified. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. Reproduce the necessary conditions and obtain results within the. Test Method Validation Acceptance Criteria.
From www.researchgate.net
Acceptance criteria applied for data evaluation. Ct cycle threshold, R Test Method Validation Acceptance Criteria It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. Matrices may be acceptable for analytical method validation. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. The choice of surrogate matrix should be scientifically justified. A validation study is designed to provide sufficient evidence that the analytical procedure. Test Method Validation Acceptance Criteria.
From www.slideserve.com
PPT Integrated Method Development and Validation PowerPoint Test Method Validation Acceptance Criteria General statement on acceptance criteria for the process. Matrices may be acceptable for analytical method validation. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. These objectives are described with. The choice of surrogate matrix should be scientifically justified. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical.. Test Method Validation Acceptance Criteria.
From www.researchgate.net
Acceptance criteria and results of the method validation parameters Test Method Validation Acceptance Criteria It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. The choice of surrogate matrix should be scientifically justified. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. Matrices may be acceptable for analytical method validation. General statement on acceptance criteria for the process. Verification of a test method. Test Method Validation Acceptance Criteria.
From www.a3p.org
Some good validation practices for analytical procedures Test Method Validation Acceptance Criteria It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Verification of a test method demonstrates that the laboratory has met the test method’s. Test Method Validation Acceptance Criteria.
From www.researchgate.net
Comparison of different guidelines for 'precision' parameter of Test Method Validation Acceptance Criteria Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. Matrices may be acceptable for analytical method validation. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. General statement on acceptance criteria. Test Method Validation Acceptance Criteria.
From www.template.net
10+ Validation Report Templates Free Sample, Example Format Download Test Method Validation Acceptance Criteria General statement on acceptance criteria for the process. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. Matrices may be acceptable for. Test Method Validation Acceptance Criteria.
From intellisoft.io
Acceptance Criteria for User Stories Check Examples & Tips Test Method Validation Acceptance Criteria General statement on acceptance criteria for the process. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. Matrices may be acceptable for analytical method validation. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. It provides guidance and recommendations on how to derive and evaluate the various. Test Method Validation Acceptance Criteria.
From www.slideserve.com
PPT A StepbyStep Guide for Method Validation PowerPoint Test Method Validation Acceptance Criteria Matrices may be acceptable for analytical method validation. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. General statement on acceptance criteria for the process. These objectives are described with. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. It provides guidance and. Test Method Validation Acceptance Criteria.
From www.lambdatest.com
Verification vs Validation Know The Differences in Testing Test Method Validation Acceptance Criteria General statement on acceptance criteria for the process. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. These objectives are described with. The choice of surrogate matrix should be scientifically justified. Verification of a test method demonstrates that the laboratory has. Test Method Validation Acceptance Criteria.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Acceptance Criteria Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. It provides guidance and recommendations on how to derive and evaluate the various. Test Method Validation Acceptance Criteria.
From www.slideserve.com
PPT Test Method Validation & Verification PowerPoint Presentation Test Method Validation Acceptance Criteria General statement on acceptance criteria for the process. The choice of surrogate matrix should be scientifically justified. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. These objectives are described with. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. A validation study is designed to provide. Test Method Validation Acceptance Criteria.
From www.amazon.com
Test Method Validation Acceptance Criteria 0602573017814 Test Method Validation Acceptance Criteria A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. General statement on acceptance criteria for the process. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. These objectives are described with.. Test Method Validation Acceptance Criteria.
From studylib.net
Establishing Acceptance Criteria for Method Validation Test Method Validation Acceptance Criteria It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. General statement on acceptance criteria for the process. These objectives are described with. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Matrices. Test Method Validation Acceptance Criteria.
From www.productplan.com
What is acceptance criteria? Definition and Best Practices Test Method Validation Acceptance Criteria These objectives are described with. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. General statement on acceptance criteria for the process. The choice of surrogate matrix should be scientifically justified. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. A validation study is designed to provide. Test Method Validation Acceptance Criteria.
From www.slideserve.com
PPT Analytical Methods What, When and How to Validate PowerPoint Test Method Validation Acceptance Criteria Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. General statement on acceptance criteria for the process. Matrices may be acceptable for analytical method validation. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. These objectives are described with. A validation study is designed to provide sufficient. Test Method Validation Acceptance Criteria.
From www.researchgate.net
Validation acceptance criteria Tablica 4 Prihvatljivi kriteriji Test Method Validation Acceptance Criteria The choice of surrogate matrix should be scientifically justified. Matrices may be acceptable for analytical method validation. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. These objectives are described with. Verification of a test method demonstrates that the. Test Method Validation Acceptance Criteria.
From lgmpharma.com
Best Practices For Successful Method Validation LGM Pharma Test Method Validation Acceptance Criteria General statement on acceptance criteria for the process. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. These objectives are described with. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. The choice of surrogate matrix should be scientifically justified. A validation study is designed to provide sufficient. Test Method Validation Acceptance Criteria.
From www.semanticscholar.org
Figure 2 from Stepbystep analytical methods validation and protocol Test Method Validation Acceptance Criteria The choice of surrogate matrix should be scientifically justified. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. These objectives are described with. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria.. Test Method Validation Acceptance Criteria.
From www.pharmaceuticalsky.com
Analytical Method Validation Protocol for Nystatin BP Test Method Validation Acceptance Criteria Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. General statement on acceptance criteria for the process. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. The choice of surrogate matrix. Test Method Validation Acceptance Criteria.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Acceptance Criteria Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. Matrices may be acceptable for analytical method validation. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. These objectives are described with. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be.. Test Method Validation Acceptance Criteria.
From theproductmanager.com
How To Write Excellent Acceptance Criteria (With Examples) The Test Method Validation Acceptance Criteria It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. General statement on acceptance criteria for the process. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. Verification of a test method demonstrates. Test Method Validation Acceptance Criteria.
From en.ppt-online.org
Method Validation and Verification Protocols for Test Methods online Test Method Validation Acceptance Criteria A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. General statement on acceptance criteria for the. Test Method Validation Acceptance Criteria.
From www.softwaretestingmaterial.com
Acceptance Testing A Complete Guide Test Method Validation Acceptance Criteria A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Matrices may be acceptable for analytical method validation. The choice of surrogate matrix should be scientifically justified. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. General statement on acceptance criteria for the process. It provides guidance and recommendations on how. Test Method Validation Acceptance Criteria.
From www.professionalqa.com
Acceptance Criteria Vs Acceptance Test Test Method Validation Acceptance Criteria General statement on acceptance criteria for the process. The choice of surrogate matrix should be scientifically justified. Matrices may be acceptable for analytical method validation. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. It provides guidance and. Test Method Validation Acceptance Criteria.
From slidetodoc.com
Establishing Validation Acceptance Criteria on the Observed Mean Test Method Validation Acceptance Criteria A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. Verification of a test method demonstrates that the laboratory has met the test method’s. Test Method Validation Acceptance Criteria.
From testorigen.com
Need and Stages of Acceptance Testing TestOrigen Test Method Validation Acceptance Criteria Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. General statement on acceptance criteria for the process. A validation study is designed to provide sufficient evidence that the analytical procedure meets its objectives. The choice of surrogate matrix should be scientifically justified. Verification of a test method demonstrates that the laboratory has met the test method’s performance. Test Method Validation Acceptance Criteria.
From informacionpublica2021.svet.gob.gt
Guide To Validation Testing Phases, Types, Importance, 48 OFF Test Method Validation Acceptance Criteria It provides guidance and recommendations on how to derive and evaluate the various validation tests for each analytical. General statement on acceptance criteria for the process. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. Matrices may be acceptable for analytical method validation. These objectives are described with. A validation study is designed to provide sufficient evidence. Test Method Validation Acceptance Criteria.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Test Method Validation Acceptance Criteria The choice of surrogate matrix should be scientifically justified. Verification of a test method demonstrates that the laboratory has met the test method’s performance specifications and must be. These objectives are described with. Reproduce the necessary conditions and obtain results within the proposed acceptance criteria. It provides guidance and recommendations on how to derive and evaluate the various validation tests. Test Method Validation Acceptance Criteria.