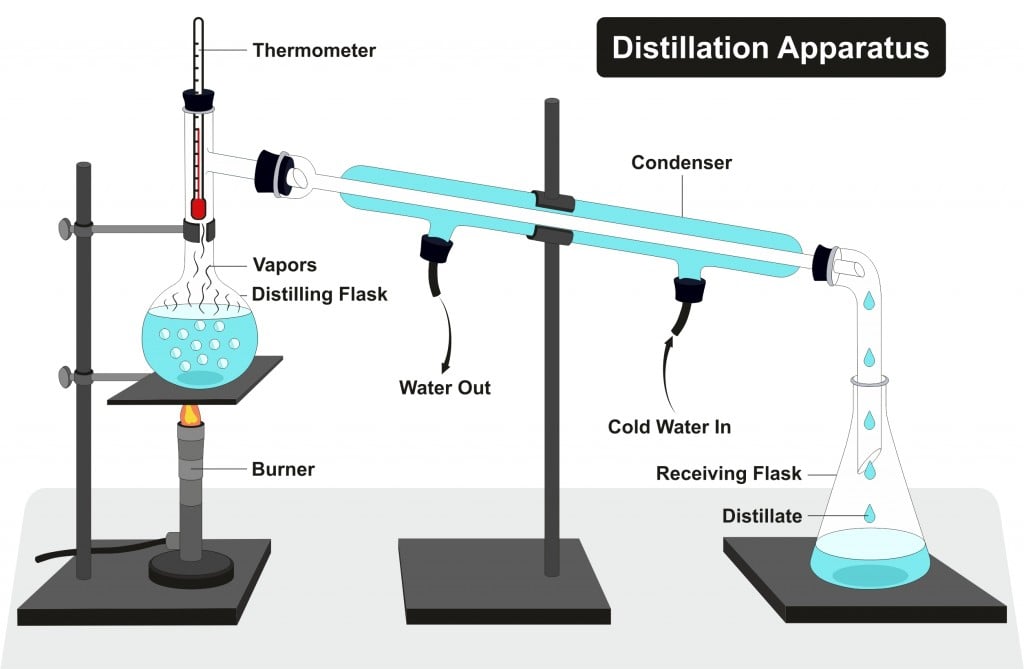
Function Of Distillation In Esterification . Mechanism of the fischer esterification. The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Addition of a proton (e.g.: Nucleophilic attack of the alcohol gives a. This is known as fischer esterification. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol.
from www.scienceabc.com
The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Mechanism of the fischer esterification. This is known as fischer esterification. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Nucleophilic attack of the alcohol gives a. Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Addition of a proton (e.g.:
Denatured Alcohol Definition, Properties, Examples And Uses
Function Of Distillation In Esterification This is known as fischer esterification. Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. This is known as fischer esterification. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. Mechanism of the fischer esterification. Nucleophilic attack of the alcohol gives a. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Addition of a proton (e.g.:
From webapi.bu.edu
💌 Fischer esterification mechanism. Fischer Esterification Definition, Examples, and Mechanism Function Of Distillation In Esterification Mechanism of the fischer esterification. Addition of a proton (e.g.: This is known as fischer esterification. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Nucleophilic attack of the alcohol gives. Function Of Distillation In Esterification.
From www.youtube.com
Esterification Reaction of alcohol YouTube Function Of Distillation In Esterification Nucleophilic attack of the alcohol gives a. Mechanism of the fischer esterification. Addition of a proton (e.g.: Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of. Function Of Distillation In Esterification.
From www.semanticscholar.org
Figure 1 from Design and control of a reactivedistillation process for esterification of an Function Of Distillation In Esterification Addition of a proton (e.g.: Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Nucleophilic attack of the alcohol gives a. Mechanism of the fischer esterification. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of. Function Of Distillation In Esterification.
From www.youtube.com
Esterification Reflux, Isolation and Purification // HSC Chemistry YouTube Function Of Distillation In Esterification The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Addition of a proton (e.g.: Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Mechanism of the fischer esterification.. Function Of Distillation In Esterification.
From www.researchgate.net
Relationship between distillation amount and esterification time in... Download Scientific Diagram Function Of Distillation In Esterification Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Mechanism of the fischer esterification. Addition of a proton (e.g.: Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. This is known as. Function Of Distillation In Esterification.
From www.researchgate.net
Distillation apparatus. Connections are either ground glass surface or... Download Scientific Function Of Distillation In Esterification Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Addition of a proton (e.g.: Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Mechanism of the fischer esterification. This is known as fischer esterification. Nucleophilic attack of the. Function Of Distillation In Esterification.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Function Of Distillation In Esterification Addition of a proton (e.g.: Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. This is known as fischer esterification. Nucleophilic attack of the alcohol gives a. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The ester can be. Function Of Distillation In Esterification.
From www.mdpi.com
Processes Free FullText Novel Process for Butyl Acetate Production via Membrane Reactor A Function Of Distillation In Esterification The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Mechanism of the fischer esterification. Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Carboxylic acids can. Function Of Distillation In Esterification.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Function Of Distillation In Esterification Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Nucleophilic attack of the alcohol gives a. This is known. Function Of Distillation In Esterification.
From www.circuitdiagram.co
Schematic Diagram Of Simple Distillation Experiment Circuit Diagram Function Of Distillation In Esterification The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. This is known as fischer esterification. Mechanism of the fischer esterification. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Addition of a proton (e.g.: Fischer esterification is the esterification of a carboxylic. Function Of Distillation In Esterification.
From www.researchgate.net
Acidcatalyzed esterification. Reaction conditions and yields for... Download Scientific Diagram Function Of Distillation In Esterification The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. Nucleophilic attack of the alcohol gives a. Carboxylic acids can be converted. Function Of Distillation In Esterification.
From www.slideserve.com
PPT Chapter 40 Esterification. Synthesis of n Butyl Acetate by Azeotropic Distillation of Function Of Distillation In Esterification The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Nucleophilic attack of the alcohol gives a. Mechanism of the fischer esterification. This is known as. Function Of Distillation In Esterification.
From www.intechopen.com
Reactive Distillation Applied to Biodiesel Production by Esterification Simulation Studies Function Of Distillation In Esterification Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Addition of a proton (e.g.: The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Mechanism of the fischer esterification. Nucleophilic attack of the alcohol gives. Function Of Distillation In Esterification.
From www.scribd.com
Process1 DirectEsterification Process Desc PDF Ester Distillation Function Of Distillation In Esterification Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. Addition of a proton (e.g.: Mechanism of the fischer esterification. Synthesis, isolation and purification of esters in a. Function Of Distillation In Esterification.
From www.semanticscholar.org
Figure 1 from Design and control of a reactivedistillation process for esterification of an Function Of Distillation In Esterification The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Addition of a proton (e.g.: Mechanism of the. Function Of Distillation In Esterification.
From www.researchgate.net
(PDF) Optimal Operation of Batch Reactive Distillation Process Involving Esterification Reaction Function Of Distillation In Esterification Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Mechanism of the fischer esterification. This is known as fischer esterification. Addition of a proton (e.g.: Nucleophilic attack of the alcohol gives a. Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and. Function Of Distillation In Esterification.
From www.chemistrysteps.com
Fischer Esterification Chemistry Steps Function Of Distillation In Esterification The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Mechanism of the fischer esterification. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Addition of a proton (e.g.:. Function Of Distillation In Esterification.
From www.slideserve.com
PPT Chapter 40 Esterification. Synthesis of n Butyl Acetate by Azeotropic Distillation of Function Of Distillation In Esterification Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Carboxylic acids can be converted to esters with an acid catalyst and an excess. Function Of Distillation In Esterification.
From www.researchgate.net
Schematic showing reactive distillation configuration. Reprinted from... Download Scientific Function Of Distillation In Esterification The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The π. Function Of Distillation In Esterification.
From www.researchgate.net
(PDF) Catalytic Reactive Distillation for the Esterification Process Experimental and Simulation Function Of Distillation In Esterification The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. Mechanism of the fischer esterification. Nucleophilic attack of the alcohol gives a. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Addition of a proton (e.g.: Synthesis,. Function Of Distillation In Esterification.
From www.scienceabc.com
Denatured Alcohol Definition, Properties, Examples And Uses Function Of Distillation In Esterification The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Addition of a proton (e.g.: Nucleophilic attack of the alcohol gives a. Mechanism of the fischer esterification. This is known as fischer esterification. Carboxylic acids can be converted to esters with an. Function Of Distillation In Esterification.
From www.animalia-life.club
Esterification Mechanism Function Of Distillation In Esterification Nucleophilic attack of the alcohol gives a. Addition of a proton (e.g.: Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. This is known as fischer esterification. Mechanism of the fischer esterification. Carboxylic acids can be converted to esters with an acid catalyst and an. Function Of Distillation In Esterification.
From www.slideserve.com
PPT Esterification PowerPoint Presentation, free download ID3150303 Function Of Distillation In Esterification Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. The ester can be separated from the carboxylic acid, alcohol,. Function Of Distillation In Esterification.
From byjus.com
Esters are sweet smelling substances and are used in making perfumes. Suggest some activity and Function Of Distillation In Esterification Nucleophilic attack of the alcohol gives a. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Mechanism of the fischer esterification. This is known as fischer esterification. Addition of a proton (e.g.: The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. The. Function Of Distillation In Esterification.
From www.semanticscholar.org
Figure 1 from Design and control of a reactivedistillation process for esterification of an Function Of Distillation In Esterification The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Nucleophilic attack of the alcohol gives a. This is known as fischer esterification. Addition of a proton (e.g.: Fischer esterification is the esterification of a carboxylic acid by heating it with an. Function Of Distillation In Esterification.
From lasopabk587.weebly.com
Vacuum Distillation Important Factors lasopabk Function Of Distillation In Esterification Addition of a proton (e.g.: This is known as fischer esterification. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. The ester can be separated from the carboxylic acid,. Function Of Distillation In Esterification.
From www.semanticscholar.org
[PDF] Catalytic Reactive Distillation for the Esterification Process Experimental and Function Of Distillation In Esterification Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Carboxylic acids can be converted to esters with an acid catalyst and an excess. Function Of Distillation In Esterification.
From www.semanticscholar.org
Figure 1 from Esterification of fatty acids in a thermally coupled reactive distillation column Function Of Distillation In Esterification Mechanism of the fischer esterification. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Nucleophilic attack of the alcohol gives a. The ester can be separated from the carboxylic acid, alcohol,. Function Of Distillation In Esterification.
From medium.com
‘Ultrapure Water and Distilled Water — What is the Difference? by Sigma Aldrich Labwater Medium Function Of Distillation In Esterification Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Synthesis, isolation and purification of esters in a direct esterification. Function Of Distillation In Esterification.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Function Of Distillation In Esterification Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in. Function Of Distillation In Esterification.
From www.vrogue.co
Pdf Catalytic Reactive Distillation For The Esterific vrogue.co Function Of Distillation In Esterification The ester can be separated from the carboxylic acid, alcohol, water and sulphuric acid in the mixture by fractional distillation. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Carboxylic acids can be converted to esters with an acid catalyst and. Function Of Distillation In Esterification.
From www.mdpi.com
Catalysts Free FullText A Thermodynamic and Study on Electrochemical Esterification Function Of Distillation In Esterification This is known as fischer esterification. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Nucleophilic attack of the alcohol gives a. Addition of a proton (e.g.: Mechanism of the fischer esterification. Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and. Function Of Distillation In Esterification.
From www.vernier.com
Fractional Distillation of Esters > Experiment 9 from Organic Chemistry with Vernier Function Of Distillation In Esterification The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the electrons from the electronegativity difference. Synthesis, isolation and purification of esters in a direct esterification reaction using an alcohol and a carboxylic acid tutorial with experimental procedures tutorial for. Nucleophilic attack of the alcohol gives a. The. Function Of Distillation In Esterification.
From www.scribd.com
Distillation Equipment Parts and Functions Distillation Physical Sciences Function Of Distillation In Esterification This is known as fischer esterification. Mechanism of the fischer esterification. Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Addition of a proton (e.g.: Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Nucleophilic attack of the alcohol gives. Function Of Distillation In Esterification.
From www.researchgate.net
Process flowsheet for RD column of free fatty acid esterification. Download Scientific Diagram Function Of Distillation In Esterification Carboxylic acids can be converted to esters with an acid catalyst and an excess of alcohol. Mechanism of the fischer esterification. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. This is known as fischer esterification. Nucleophilic attack of the alcohol gives a. The π bond of. Function Of Distillation In Esterification.