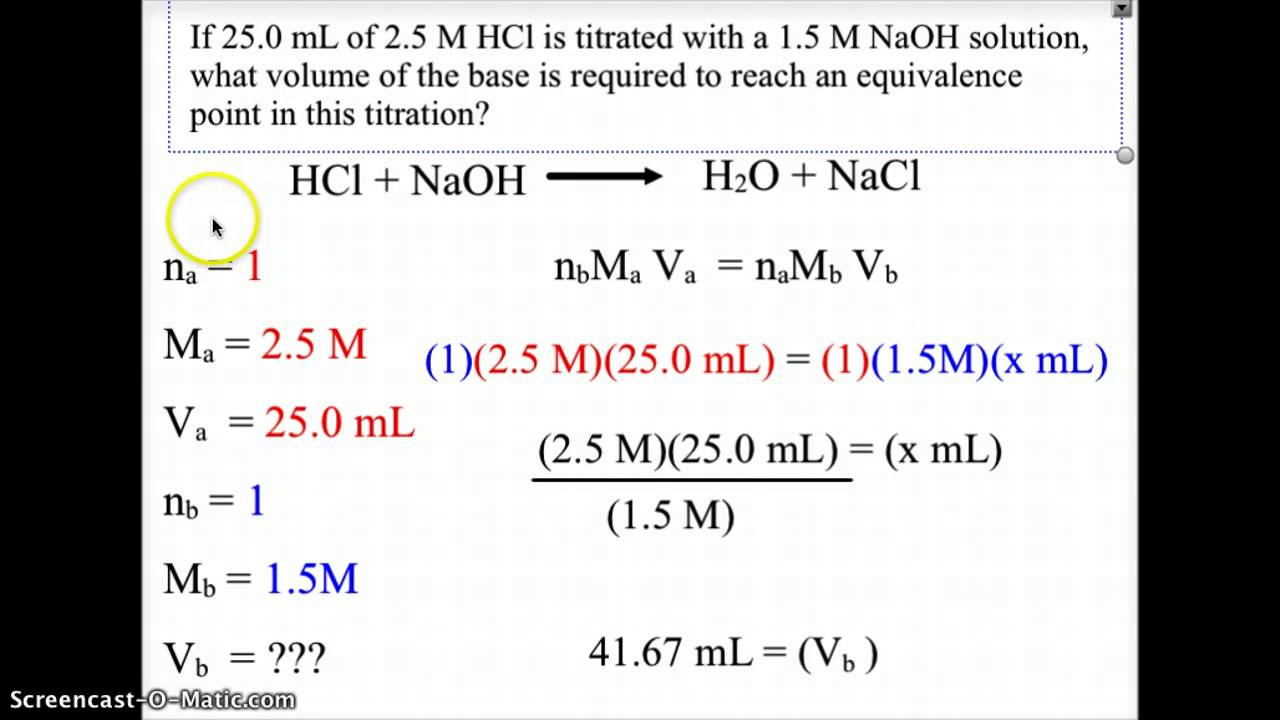
End Point Titration Formula . In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Using the initial and final reading, the volume added can be determined quite precisely: The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. Suppose that a titration is performed and \(20.70 \: Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. You will also learn about equivalence points and. With an indicator the precision of the end point signal is usually. After the titration has reached the endpoint, a final volume is read from the buret. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. \ce{naoh}\) is required to reach the end point when titrated.
from www.youtube.com
Using the initial and final reading, the volume added can be determined quite precisely: Using a ph probe and using a colour indicator. In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Suppose that a titration is performed and \(20.70 \: For a ph titration (between an acid and a base), there are two common ways to find the endpoint: \ce{naoh}\) is required to reach the end point when titrated. Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. With an indicator the precision of the end point signal is usually. You will also learn about equivalence points and.
AcidBase Titration Equivalence Point YouTube
End Point Titration Formula In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. With an indicator the precision of the end point signal is usually. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: \ce{naoh}\) is required to reach the end point when titrated. The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. You will also learn about equivalence points and. Suppose that a titration is performed and \(20.70 \: Using a ph probe and using a colour indicator. In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download End Point Titration Formula For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using a ph probe and using a colour indicator. After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: In this tutorial,. End Point Titration Formula.
From ar.inspiredpencil.com
Endpoint Titration End Point Titration Formula Using a ph probe and using a colour indicator. Using the initial and final reading, the volume added can be determined quite precisely: Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. \ce{naoh}\) is required to reach the end point when titrated. You will also learn about equivalence points. End Point Titration Formula.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki End Point Titration Formula Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. Using the initial and final reading, the volume added can be determined quite precisely: With an. End Point Titration Formula.
From hxejxcnlz.blob.core.windows.net
Indicator Use In Lodimetric Titration at Patricia Jolley blog End Point Titration Formula You will also learn about equivalence points and. After the titration has reached the endpoint, a final volume is read from the buret. With an indicator the precision of the end point signal is usually. The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. In. End Point Titration Formula.
From mmerevise.co.uk
Titrations and Uncertainties MME End Point Titration Formula Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. Suppose that a titration is performed and \(20.70 \: In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Using a ph probe and using a colour indicator. The precision of detecting. End Point Titration Formula.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube End Point Titration Formula You will also learn about equivalence points and. The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. After the titration has reached the endpoint, a. End Point Titration Formula.
From www.youtube.com
Midpoint, Distance, and Endpoint Formulas YouTube End Point Titration Formula In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. After the titration has reached the endpoint, a final volume is read from the buret. Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. For a ph titration (between an acid. End Point Titration Formula.
From www.inchcalculator.com
Endpoint Calculator Inch Calculator End Point Titration Formula You will also learn about equivalence points and. After the titration has reached the endpoint, a final volume is read from the buret. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The precision of detecting the end point depends on how it is measured and the slope of the. End Point Titration Formula.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation End Point Titration Formula With an indicator the precision of the end point signal is usually. For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Suppose that a titration is performed and \(20.70 \: In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will. End Point Titration Formula.
From www.animalia-life.club
Endpoint Titration End Point Titration Formula After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: Using a ph probe and using a colour indicator. With an indicator the precision of the end point signal is usually. Suppose that a titration is performed and \(20.70 \: The. End Point Titration Formula.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple End Point Titration Formula With an indicator the precision of the end point signal is usually. Using the initial and final reading, the volume added can be determined quite precisely: Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. You will also learn about equivalence points and. For a ph titration (between an. End Point Titration Formula.
From studygripewater.z21.web.core.windows.net
How To Find The Other Endpoint Formula End Point Titration Formula The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. Using a ph probe and using a colour indicator. Suppose that a titration is performed and \(20.70 \: With an indicator the precision of the end point signal is usually. You will also learn about equivalence. End Point Titration Formula.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 End Point Titration Formula You will also learn about equivalence points and. With an indicator the precision of the end point signal is usually. After the titration has reached the endpoint, a final volume is read from the buret. \ce{naoh}\) is required to reach the end point when titrated. Using a ph probe and using a colour indicator. The precision of detecting the end. End Point Titration Formula.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas End Point Titration Formula After the titration has reached the endpoint, a final volume is read from the buret. Suppose that a titration is performed and \(20.70 \: The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. With an indicator the precision of the end point signal is usually.. End Point Titration Formula.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog End Point Titration Formula Suppose that a titration is performed and \(20.70 \: In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. Using the initial and final reading, the volume added can be determined quite. End Point Titration Formula.
From giotpzhko.blob.core.windows.net
Radius And Endpoints at Mary Bohr blog End Point Titration Formula Using the initial and final reading, the volume added can be determined quite precisely: For a ph titration (between an acid and a base), there are two common ways to find the endpoint: The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. Titration is a. End Point Titration Formula.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations End Point Titration Formula In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. Using the initial and final reading, the volume added can be determined quite precisely: For a ph titration (between an acid and. End Point Titration Formula.
From shimidoon.ir
تیتراسیون چیست؟ چگونه انجام می شود؟ * شیمی دون End Point Titration Formula In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. With an indicator the precision of the end point signal is usually. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. After the titration has reached the endpoint, a final volume is. End Point Titration Formula.
From es.wikihow.com
Cómo hacer una titulación (con imágenes) wikiHow End Point Titration Formula In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn about equivalence points and. Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. Using the initial and final reading, the volume added can be determined quite precisely:. End Point Titration Formula.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 End Point Titration Formula Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. After the titration has reached the endpoint, a final volume is read from the buret. With an indicator the precision of the end point signal is usually. For a ph titration (between an acid and a base), there are two common. End Point Titration Formula.
From theedge.com.hk
Chemistry How To Titration The Edge End Point Titration Formula Using the initial and final reading, the volume added can be determined quite precisely: \ce{naoh}\) is required to reach the end point when titrated. Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. Suppose that a titration is performed and \(20.70 \: The precision of detecting the end point. End Point Titration Formula.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co End Point Titration Formula In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. Using the initial and final reading, the volume added can be determined quite precisely: Suppose that a titration. End Point Titration Formula.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts End Point Titration Formula For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Suppose that a titration is performed and \(20.70 \: After the titration has reached the endpoint, a final volume is read from the buret. Using a ph probe and using a colour indicator. In this tutorial, you will learn about titration. End Point Titration Formula.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest End Point Titration Formula With an indicator the precision of the end point signal is usually. Using the initial and final reading, the volume added can be determined quite precisely: The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. In this tutorial, you will learn about titration curves, titration. End Point Titration Formula.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS End Point Titration Formula For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. You will also learn about equivalence points and. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to. End Point Titration Formula.
From chem.libretexts.org
3.7 AcidBase Titrations Chemistry LibreTexts End Point Titration Formula Using a ph probe and using a colour indicator. Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. After the titration has reached the endpoint, a final volume is read from the buret. Using the initial and final reading, the volume added can be determined quite precisely: With an. End Point Titration Formula.
From hxenjakty.blob.core.windows.net
Titration Types Definition at John Justice blog End Point Titration Formula You will also learn about equivalence points and. After the titration has reached the endpoint, a final volume is read from the buret. Suppose that a titration is performed and \(20.70 \: Using a ph probe and using a colour indicator. The precision of detecting the end point depends on how it is measured and the slope of the titration. End Point Titration Formula.
From mungfali.com
Acid Base Titration Procedure End Point Titration Formula The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. Suppose that a titration is performed and \(20.70 \: Using a ph probe and using a colour indicator. You will also learn about equivalence points and. In this tutorial, you will learn about titration curves, titration. End Point Titration Formula.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记1.7.2 Titrations翰林国际教育 End Point Titration Formula The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. Suppose that a titration is performed and \(20.70 \: Using the initial and final reading, the volume added can be determined quite precisely: Using a ph probe and using a colour indicator. You will also learn. End Point Titration Formula.
From morioh.com
Acid Base Titration Curves PH Calculations End Point Titration Formula The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. Using the initial and final reading, the volume added can be determined quite precisely: You will also learn about equivalence points and. Suppose that a titration is performed and \(20.70 \: Using a ph probe and. End Point Titration Formula.
From giorlbtcj.blob.core.windows.net
How To Calculate Molar Concentration In Titration at Tessie Donato blog End Point Titration Formula The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. With an indicator the precision of the end point signal is usually. After the titration has reached the endpoint, a final volume is read from the buret. For a ph titration (between an acid and a. End Point Titration Formula.
From www.brainkart.com
Selecting and Evaluating the End Point Titrations Based on Redox End Point Titration Formula Using the initial and final reading, the volume added can be determined quite precisely: In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Using a ph probe and using a colour indicator. For a ph titration (between an acid and a base), there are two common ways to find the endpoint:. End Point Titration Formula.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators End Point Titration Formula For a ph titration (between an acid and a base), there are two common ways to find the endpoint: Using the initial and final reading, the volume added can be determined quite precisely: \ce{naoh}\) is required to reach the end point when titrated. After the titration has reached the endpoint, a final volume is read from the buret. In this. End Point Titration Formula.
From www.youtube.com
Acid Base Titrations, pH Curves and Endpoints YouTube End Point Titration Formula With an indicator the precision of the end point signal is usually. The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. Titration is a common laboratory technique used to determine the concentration of one chemical compound or analyte based on its. Suppose that a titration. End Point Titration Formula.
From studydystrophic.z4.web.core.windows.net
How To Find Equivalence Point On Excel Graph End Point Titration Formula \ce{naoh}\) is required to reach the end point when titrated. The precision of detecting the end point depends on how it is measured and the slope of the titration curve at the end point. In this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Using the initial and final reading, the volume. End Point Titration Formula.