Phosphate Buffer Hplc . Typically, i was running the hplc for weeks. Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. When i changed to a smaller. The buffer was 50 mm phosphate, 150 mm nacl, ph 8.5. To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic component (usually kh 2 po 4) to. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the.
from helixchrom.com
When i changed to a smaller. Typically, i was running the hplc for weeks. To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic component (usually kh 2 po 4) to. For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. The buffer was 50 mm phosphate, 150 mm nacl, ph 8.5. Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the.
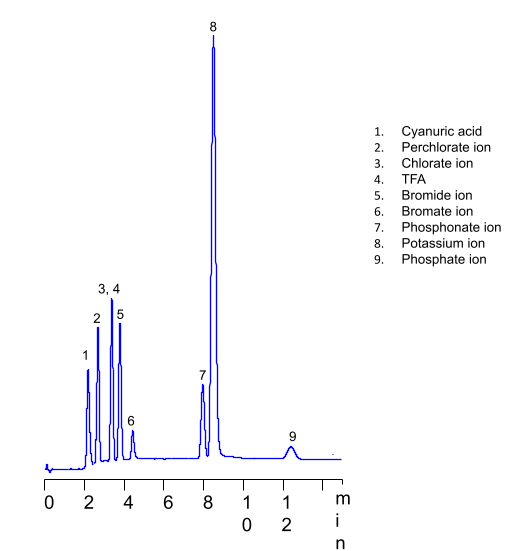
HPLC Methods for analysis of Phosphate Ion HELIX Chromatography
Phosphate Buffer Hplc The buffer was 50 mm phosphate, 150 mm nacl, ph 8.5. Typically, i was running the hplc for weeks. For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic component (usually kh 2 po 4) to. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. When i changed to a smaller. The buffer was 50 mm phosphate, 150 mm nacl, ph 8.5.
From www.youtube.com
phosphate buffer preparation protocol phosphate buffer as per usp Phosphate Buffer Hplc This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. The buffer was 50 mm phosphate, 150 mm nacl, ph 8.5. When i changed to a smaller. Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue.. Phosphate Buffer Hplc.
From www.semanticscholar.org
Figure 2 from Optimization of Phosphate Buffer Ph 4.4Methanol as Phosphate Buffer Hplc Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. When. Phosphate Buffer Hplc.
From www.cephamls.com
Phosphate Buffer, pH 8.5 [0.5 M] Cepham Life Sciences Research Products Phosphate Buffer Hplc Typically, i was running the hplc for weeks. For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological. Phosphate Buffer Hplc.
From www.cephamls.com
Phosphate Buffer, pH 8.0 [0.5 M] Cepham Life Sciences Research Products Phosphate Buffer Hplc Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent. Phosphate Buffer Hplc.
From www.researchgate.net
HPLCFLD chromatograms of extracted samples phosphate buffer solution Phosphate Buffer Hplc This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. Knowing the solubility of a given buffer. Phosphate Buffer Hplc.
From www.researchgate.net
Confirmation of bioconjugation by cation exchange HPLC. Buffer A 10 mM Phosphate Buffer Hplc Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue. Typically, i was running the hplc for weeks. To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic component (usually kh 2 po 4) to. Modern hplc method development is dominated by a. Phosphate Buffer Hplc.
From www.youtube.com
Reversed Phase HPLC 28 How Buffers Work YouTube Phosphate Buffer Hplc Typically, i was running the hplc for weeks. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue. The problem of salt precipitation in hplc applications is. Phosphate Buffer Hplc.
From www.researchgate.net
RPHPLC chromatograms of monoALDPEG2Koctreotides incubated in 0.1 M Phosphate Buffer Hplc Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris,. Phosphate Buffer Hplc.
From fdneurotech.com
0.1 M Phosphate Buffer, pH 7.4 FD Neuro Technologies, Inc. Phosphate Buffer Hplc Typically, i was running the hplc for weeks. Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue. To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic component (usually kh 2 po 4) to. This month we take a look at the. Phosphate Buffer Hplc.
From www.researchgate.net
HPLC profiles of rutin solutions (1 mM) treated in phosphate buffer, pH Phosphate Buffer Hplc Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. To form a phosphate buffer, we mix. Phosphate Buffer Hplc.
From labmal.com
Phosphate buffered saline (SigmaAldrich) LabMal Phosphate Buffer Hplc The buffer was 50 mm phosphate, 150 mm nacl, ph 8.5. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. Typically, i was running the hplc for weeks. Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome. Phosphate Buffer Hplc.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Phosphate Buffer Hplc The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. To form a phosphate buffer, we mix. Phosphate Buffer Hplc.
From sielc.com
HPLC Separation of Catecholamines on Primesep 100 Column with Phosphate Phosphate Buffer Hplc Typically, i was running the hplc for weeks. For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological. Phosphate Buffer Hplc.
From www.researchgate.net
RadioHPLC profiles of [ 18 F]5 after incubation in phosphate buffer Phosphate Buffer Hplc When i changed to a smaller. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. Modern hplc method development is dominated by a. Phosphate Buffer Hplc.
From www.researchgate.net
HPLC chromatogram (isocratic, 0.05 mol/L phosphate buffer (pH = 7.0 Phosphate Buffer Hplc For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. When i changed to a smaller. Typically, i was running the hplc for weeks. To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic component (usually kh 2 po 4) to. The buffer was 50 mm phosphate, 150 mm. Phosphate Buffer Hplc.
From www.youtube.com
prepare HPLC buffer solution phosphates buffer acetate buffer HPLC Phosphate Buffer Hplc Typically, i was running the hplc for weeks. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue. To form a phosphate buffer, we mix an acidic. Phosphate Buffer Hplc.
From www.researchgate.net
HPLC chromatograms of inositol phosphates. a Inositol phosphate Phosphate Buffer Hplc This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic. Phosphate Buffer Hplc.
From www.slideserve.com
PPT Unit Five The Body Fluids and Kidneys PowerPoint Presentation Phosphate Buffer Hplc To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic component (usually kh 2 po 4) to. For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the.. Phosphate Buffer Hplc.
From veeprho.com
Importance of Mobile Phase Buffer Selection for HPLC to LCMS Veeprho Phosphate Buffer Hplc For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. The buffer was 50 mm phosphate, 150 mm nacl, ph 8.5. Typically, i was running the hplc for weeks. The problem of salt. Phosphate Buffer Hplc.
From www.scribd.com
Prepare HPLC Buffers PDF High Performance Liquid Chromatography Phosphate Buffer Hplc For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. When i changed to a smaller. To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic component. Phosphate Buffer Hplc.
From www.semanticscholar.org
Figure 2 from A Guide to HPLC & LCMS Buffer Selectionby John W Dolan Phosphate Buffer Hplc Typically, i was running the hplc for weeks. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the. Phosphate Buffer Hplc.
From www.researchgate.net
(A) SEHPLC of Advate (400 IU/mL) eluted with 20 mM phosphate buffer Phosphate Buffer Hplc When i changed to a smaller. Typically, i was running the hplc for weeks. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. This month we take a look at the important topic of buffer choice for hplc. Phosphate Buffer Hplc.
From helixchrom.com
HPLC Methods for analysis of Phosphate Ion HELIX Chromatography Phosphate Buffer Hplc The buffer was 50 mm phosphate, 150 mm nacl, ph 8.5. To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic component (usually kh 2 po 4) to. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high. Phosphate Buffer Hplc.
From www.youtube.com
A Guide For Selection of Buffer for HPLC YouTube Phosphate Buffer Hplc Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. When i changed to a smaller. Typically, i was running the hplc for weeks. Knowing the solubility of a given buffer in the. Phosphate Buffer Hplc.
From www.researchgate.net
HPLC chromatograms (C18 5m column; acetonitrilesodium phosphate buffer Phosphate Buffer Hplc The buffer was 50 mm phosphate, 150 mm nacl, ph 8.5. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. When i changed to a smaller. For this reason, the inorganic buffers like phosphate salts does not work. Phosphate Buffer Hplc.
From www.semanticscholar.org
Figure 1 from Optimization of Phosphate Buffer Ph 4.4Methanol as Phosphate Buffer Hplc Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris,. Phosphate Buffer Hplc.
From sielc.com
Buffer Preparation SIELC Technologies Phosphate Buffer Hplc The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. The buffer was 50 mm phosphate, 150 mm nacl, ph 8.5. To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic component (usually. Phosphate Buffer Hplc.
From www.researchgate.net
HPLC chromatograms (C18 5m column; acetonitrilesodium phosphate buffer Phosphate Buffer Hplc The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. Knowing the solubility of a given buffer. Phosphate Buffer Hplc.
From sielc.com
Phosphate SIELC Phosphate Buffer Hplc Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue. Typically, i was running the hplc for weeks. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content.. Phosphate Buffer Hplc.
From www.researchgate.net
HPLC monitoring of the reaction of ZnL HSP (35 mM) in a phosphate Phosphate Buffer Hplc Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers. Phosphate Buffer Hplc.
From sielc.com
HPLC Method for Analysis of Ascorbyl Phosphate on Newcrom BH Column Phosphate Buffer Hplc To form a phosphate buffer, we mix an acidic component (usually phosphoric acid) and a basic component (usually kh 2 po 4) to. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological buffers (tris, hips, mops, etc.) in a solvent mix of high or rapidly increasing organic content. For this reason, the inorganic buffers. Phosphate Buffer Hplc.
From www.labdepotinc.com
Ammonium Phosphate Monobasic Crystalline HPLC Grade Phosphate Buffer Hplc This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. The buffer was 50 mm phosphate, 150 mm nacl, ph 8.5. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. The problem of salt precipitation. Phosphate Buffer Hplc.
From www.researchgate.net
(PDF) Physicochemical modelling of solute retention in reversedphase Phosphate Buffer Hplc Knowing the solubility of a given buffer in the respective mobile phase composition is a simple way to overcome this issue. For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. The problem. Phosphate Buffer Hplc.
From www.researchgate.net
area under curve of peak representing OP in 0.1 M sodium phosphate Phosphate Buffer Hplc This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. Modern hplc method development is dominated by a small number of ph adjusting reagents and/or buffers, that are prevalent even when the. Knowing the solubility of a given buffer in the respective mobile phase composition is a simple. Phosphate Buffer Hplc.
From www.researchgate.net
Chromatograms of (a) isotonic phosphate buffer pH 7.2; (b) 20 Phosphate Buffer Hplc For this reason, the inorganic buffers like phosphate salts does not work with mass spectrometer. This month we take a look at the important topic of buffer choice for hplc separations, how to the choose the correct. Typically, i was running the hplc for weeks. The problem of salt precipitation in hplc applications is common with phosphate buffers or biological. Phosphate Buffer Hplc.