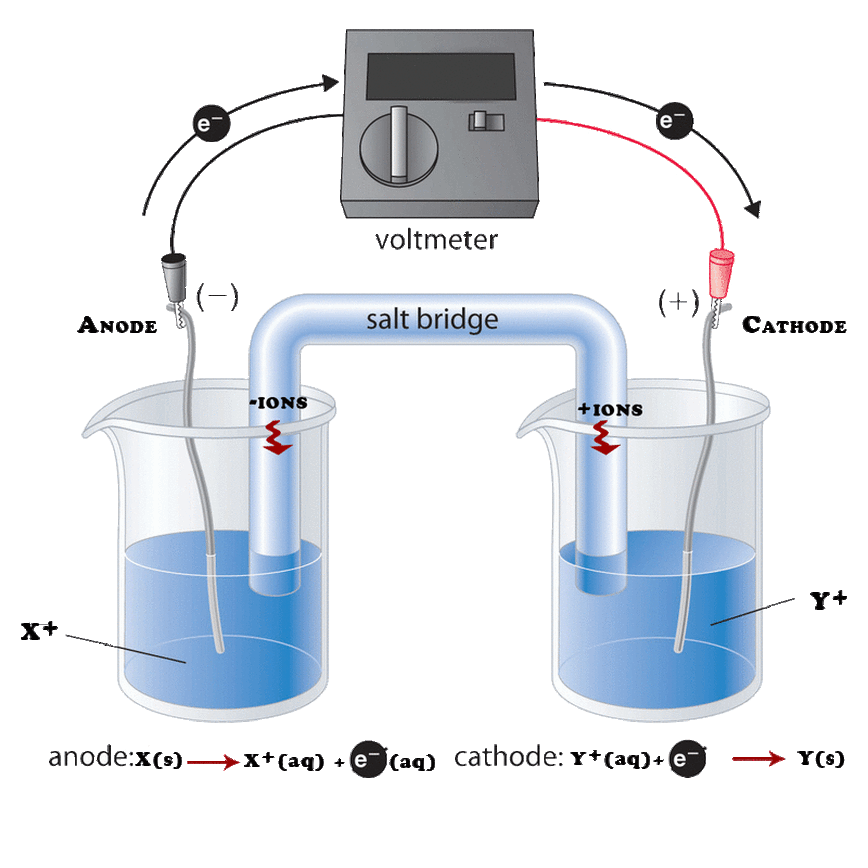
A Galvanic Cell The Salt Bridge . a salt bridge plays an important role in a galvanic cell. the salt bridge is a vital component of any voltaic cell. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. The salt bridge consists of a concentrated, nonreactive, electrolyte. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). Let us see what happens if there is no salt bridge present in the galvanic cell. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. the circuit is closed using a salt bridge, which transmits the current with moving ions.
from biochemreview.weebly.com
the circuit is closed using a salt bridge, which transmits the current with moving ions. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). the salt bridge is a vital component of any voltaic cell. Let us see what happens if there is no salt bridge present in the galvanic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte. a salt bridge plays an important role in a galvanic cell.
Galvanic cell BIOChemReview
A Galvanic Cell The Salt Bridge the circuit is closed using a salt bridge, which transmits the current with moving ions. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). the circuit is closed using a salt bridge, which transmits the current with moving ions. the salt bridge is a vital component of any voltaic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. Let us see what happens if there is no salt bridge present in the galvanic cell. a salt bridge plays an important role in a galvanic cell.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and A Galvanic Cell The Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. Its purpose is to keep the electrochemical reaction from. A Galvanic Cell The Salt Bridge.
From www.youtube.com
In a galvanic cell, the salt bridge YouTube A Galvanic Cell The Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte. the circuit is closed using a salt bridge, which transmits the current with moving ions. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). Let us see what happens. A Galvanic Cell The Salt Bridge.
From handwiki.org
ChemistrySalt bridge HandWiki A Galvanic Cell The Salt Bridge the circuit is closed using a salt bridge, which transmits the current with moving ions. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. a salt bridge plays an important role in a galvanic cell. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). the. A Galvanic Cell The Salt Bridge.
From www.vrogue.co
Galvanic Cell Salt Bridge vrogue.co A Galvanic Cell The Salt Bridge Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. The salt bridge consists of a concentrated, nonreactive, electrolyte. a salt bridge plays an important role in a galvanic cell. Let us see what happens if there is no salt bridge present in the galvanic cell. The purpose of the salt bridge is to keep the. A Galvanic Cell The Salt Bridge.
From ar.inspiredpencil.com
Salt Bridge A Galvanic Cell The Salt Bridge a salt bridge plays an important role in a galvanic cell. the salt bridge is a vital component of any voltaic cell. the circuit is closed using a salt bridge, which transmits the current with moving ions. Let us see what happens if there is no salt bridge present in the galvanic cell. It is a tube. A Galvanic Cell The Salt Bridge.
From www.youtube.com
What is a galvanic cell ? Draw its labelled diagram and explain the A Galvanic Cell The Salt Bridge Let us see what happens if there is no salt bridge present in the galvanic cell. the salt bridge is a vital component of any voltaic cell. the circuit is closed using a salt bridge, which transmits the current with moving ions. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. The salt bridge. A Galvanic Cell The Salt Bridge.
From www.numerade.com
SOLVED 'QUESTIONA Draw the following galvanic cell (salt bridge design A Galvanic Cell The Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. a salt bridge plays an important role in a galvanic cell. It is a tube filled with an electrolyte solution such as kno 3 (s). A Galvanic Cell The Salt Bridge.
From www.echemi.com
Where do negative ions migrate from the salt bridge to which electrode A Galvanic Cell The Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). a salt bridge plays an important role in. A Galvanic Cell The Salt Bridge.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working A Galvanic Cell The Salt Bridge the circuit is closed using a salt bridge, which transmits the current with moving ions. The salt bridge consists of a concentrated, nonreactive, electrolyte. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). Let us see what happens if there is no salt bridge present in the galvanic cell. Its purpose. A Galvanic Cell The Salt Bridge.
From circuitdbnighters.z13.web.core.windows.net
Salt Bridge Diagram A Galvanic Cell The Salt Bridge The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). a salt bridge plays an important role in a galvanic cell. the salt bridge is a. A Galvanic Cell The Salt Bridge.
From www.youtube.com
Lec21 Galvanic Cell or Voltaic Cell Salt Bridge Representation of A Galvanic Cell The Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte. the salt bridge is a vital component of any voltaic cell. Let us see what happens if there is no salt bridge present in the galvanic cell. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. a salt bridge plays an important role in a. A Galvanic Cell The Salt Bridge.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download A Galvanic Cell The Salt Bridge a salt bridge plays an important role in a galvanic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte. the circuit is closed using a salt bridge, which transmits the current with moving ions. the salt bridge is a vital component of any voltaic cell. The purpose of the salt bridge is to keep the solutions. A Galvanic Cell The Salt Bridge.
From stock.adobe.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and A Galvanic Cell The Salt Bridge The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. The salt bridge consists of a concentrated, nonreactive, electrolyte. Let us see what happens if there is no salt bridge present in the galvanic cell. the salt bridge is a vital component of any. A Galvanic Cell The Salt Bridge.
From www.youtube.com
Daniell_Galvanic_Voltaic Cell 👉 Salt Bridge 👉 Equilibrium constant K A Galvanic Cell The Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. the circuit is closed using a salt bridge, which transmits the current with moving ions. Its purpose is to keep the electrochemical reaction from reaching. A Galvanic Cell The Salt Bridge.
From www.vecteezy.com
Electrochemical cell or Galvanic cell with Voltmeter and the function A Galvanic Cell The Salt Bridge Let us see what happens if there is no salt bridge present in the galvanic cell. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. the. A Galvanic Cell The Salt Bridge.
From www.thoughtco.com
How Galvanic or Voltaic Cells Work A Galvanic Cell The Salt Bridge a salt bridge plays an important role in a galvanic cell. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. It is a tube filled with an electrolyte solution such. A Galvanic Cell The Salt Bridge.
From diagramdatadecolour.z21.web.core.windows.net
Salt Bridge Diagram A Galvanic Cell The Salt Bridge Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. the salt bridge is a vital component of any voltaic cell. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. It is a tube filled with an electrolyte solution such. A Galvanic Cell The Salt Bridge.
From exomvxjlq.blob.core.windows.net
The Galvanic Cell Described By at Geraldo Walls blog A Galvanic Cell The Salt Bridge The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. a salt bridge plays an important role in a galvanic cell. Let us see what happens if there is no salt. A Galvanic Cell The Salt Bridge.
From www.youtube.com
Galvanic cell, Electrodes used in galvanic cell & Salt Bridge A Galvanic Cell The Salt Bridge a salt bridge plays an important role in a galvanic cell. Let us see what happens if there is no salt bridge present in the galvanic cell. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The purpose of the salt bridge is to keep the solutions electrically neutral and allow. A Galvanic Cell The Salt Bridge.
From leaderland.academy
Salt Bridge Wikipedia, 43 OFF leaderland.academy A Galvanic Cell The Salt Bridge Let us see what happens if there is no salt bridge present in the galvanic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. the circuit is closed using a salt bridge, which. A Galvanic Cell The Salt Bridge.
From www.freepik.com
Premium Vector Galvanic cell diagram cu zn salt bridge cuso4 znso4 A Galvanic Cell The Salt Bridge Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. The salt bridge consists of a concentrated, nonreactive, electrolyte. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from. A Galvanic Cell The Salt Bridge.
From biochemreview.weebly.com
Galvanic cell BIOChemReview A Galvanic Cell The Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte. the circuit is closed using a salt bridge, which transmits the current with moving ions. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one. A Galvanic Cell The Salt Bridge.
From www.youtube.com
ELECTROCHEMICAL CELL. GALVANIC CELL. SALT BRIDGE. ELECTRODE POTENTIAL A Galvanic Cell The Salt Bridge the salt bridge is a vital component of any voltaic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. Let us see what happens if there is no salt bridge present in the galvanic cell. It is a tube filled with an electrolyte solution such. A Galvanic Cell The Salt Bridge.
From mavink.com
Salt Bridge Electrochemical Cell A Galvanic Cell The Salt Bridge Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. a salt bridge plays an important role in a galvanic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte. It. A Galvanic Cell The Salt Bridge.
From www.chegg.com
Solved salt bridge 1 Construct a galvanic (voltaic) cell A Galvanic Cell The Salt Bridge Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. the salt bridge is a vital component of any voltaic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). Let us see what happens if there is no. A Galvanic Cell The Salt Bridge.
From www.youtube.com
ELECTRO CHEMISTRY/CLASS 12/PART3/GALVANIC CELL/SALT BRIDGE. YouTube A Galvanic Cell The Salt Bridge It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). Let us see what happens if there is no salt bridge present in the galvanic cell. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. the. A Galvanic Cell The Salt Bridge.
From www.youtube.com
KAC32.17 Electrochemistry The Role of the Salt Bridge YouTube A Galvanic Cell The Salt Bridge Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. the salt bridge is a vital component of any voltaic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. . A Galvanic Cell The Salt Bridge.
From www.numerade.com
SOLVED HW27.2 Galvanic Cells 2 Voltmeter and Salt Bridge Calculate A Galvanic Cell The Salt Bridge It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). the circuit is closed using a salt bridge, which transmits the current with moving ions. The salt bridge consists of a concentrated, nonreactive, electrolyte. Let us see what happens if there is no salt bridge present in the galvanic cell. a. A Galvanic Cell The Salt Bridge.
From www.chegg.com
Solved To construct the galvanic cell illustrated above, the A Galvanic Cell The Salt Bridge Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. a salt bridge plays an important role in a galvanic cell. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. the circuit is closed using a salt bridge, which. A Galvanic Cell The Salt Bridge.
From www.youtube.com
Galvanic Cell Or Voltaic Cell Salt Bridge Animation Chemistry Ask A Galvanic Cell The Salt Bridge the circuit is closed using a salt bridge, which transmits the current with moving ions. Let us see what happens if there is no salt bridge present in the galvanic cell. a salt bridge plays an important role in a galvanic cell. the salt bridge is a vital component of any voltaic cell. Its purpose is to. A Galvanic Cell The Salt Bridge.
From www.youtube.com
The purpose of the salt bridge in a galvanic cell is to YouTube A Galvanic Cell The Salt Bridge the circuit is closed using a salt bridge, which transmits the current with moving ions. the salt bridge is a vital component of any voltaic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte. Let us see what happens if there is no salt bridge present in the galvanic cell. a salt bridge plays an important. A Galvanic Cell The Salt Bridge.
From www.reddit.com
Galvanic cell salt bridges Mcat A Galvanic Cell The Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte. a salt bridge plays an important role in a galvanic cell. the circuit is closed using a salt bridge, which transmits the current with moving ions. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). the salt bridge is a. A Galvanic Cell The Salt Bridge.
From byjus.com
Salt Bridge Definition, Function, Types, Preparation, Galvanic Cells A Galvanic Cell The Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell to another. Let us see what happens if there is no salt bridge present in the galvanic cell. a salt bridge plays an important role in a. A Galvanic Cell The Salt Bridge.
From studiousguy.com
Examples of Galvanic Cells in Everyday Life StudiousGuy A Galvanic Cell The Salt Bridge Let us see what happens if there is no salt bridge present in the galvanic cell. The salt bridge consists of a concentrated, nonreactive, electrolyte. Its purpose is to keep the electrochemical reaction from reaching equilibrium too quickly. It is a tube filled with an electrolyte solution such as kno 3 (s) or kcl (s). the circuit is closed. A Galvanic Cell The Salt Bridge.
From www.youtube.com
Electrochemistry Part5 Galvanic cell Salt bridge Physical A Galvanic Cell The Salt Bridge The salt bridge consists of a concentrated, nonreactive, electrolyte. a salt bridge plays an important role in a galvanic cell. the circuit is closed using a salt bridge, which transmits the current with moving ions. The purpose of the salt bridge is to keep the solutions electrically neutral and allow the free flow of ions from one cell. A Galvanic Cell The Salt Bridge.