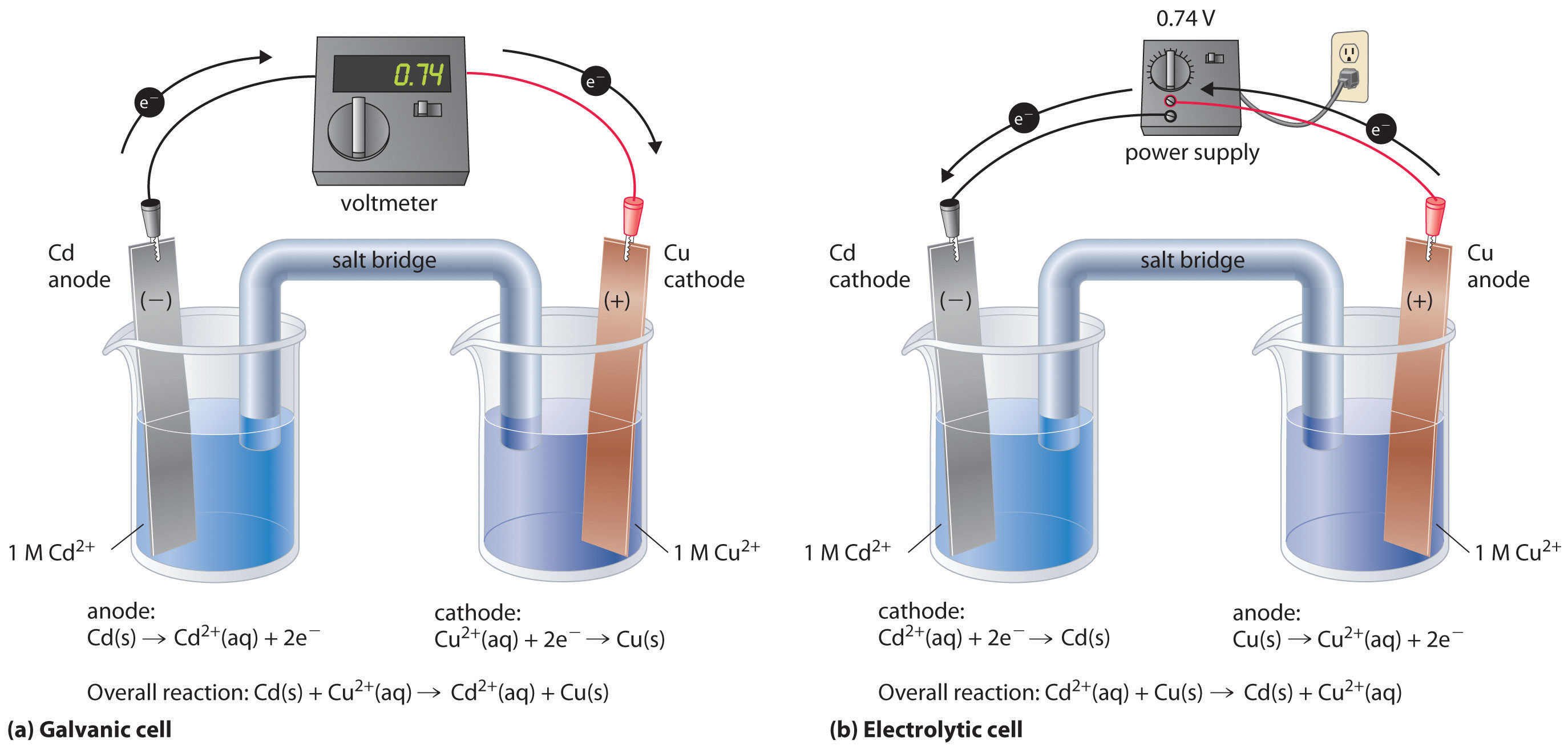
Electrochemical Cell Explained . An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. List some of the characteristics, applications and limitations of cells and batteries. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Describe the basic components of electrochemical cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Know the difference between galvanic. This kind of cell includes the galvanic, or voltaic, cell, named after.
from saylordotorg.github.io
List some of the characteristics, applications and limitations of cells and batteries. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Describe the basic components of electrochemical cells. This kind of cell includes the galvanic, or voltaic, cell, named after. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. Know the difference between galvanic. An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to.
Electrochemistry
Electrochemical Cell Explained An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after. Know the difference between galvanic. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. List some of the characteristics, applications and limitations of cells and batteries. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Describe the basic components of electrochemical cells. An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction.
From www.slideserve.com
PPT Electrochemical Cells (voltaic cells) PowerPoint Presentation Electrochemical Cell Explained An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Know the difference between galvanic. An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released. Electrochemical Cell Explained.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Electrochemical Cell Explained This kind of cell includes the galvanic, or voltaic, cell, named after. Describe the basic components of electrochemical cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction.. Electrochemical Cell Explained.
From www.youtube.com
Electrochemistry 04 Writing Electrochemical Cell Notation YouTube Electrochemical Cell Explained Know the difference between galvanic. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. List some of the characteristics, applications and limitations of cells and batteries. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a. Electrochemical Cell Explained.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemical Cell Explained An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. This kind of cell includes the galvanic, or voltaic, cell, named after. Describe the basic components of electrochemical cells.. Electrochemical Cell Explained.
From www.thoughtco.com
Electrochemical Cell Definition Electrochemical Cell Explained This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Describe the basic components of electrochemical cells. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. An electrochemical cell is. Electrochemical Cell Explained.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses Electrochemical Cell Explained This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. Know the difference between galvanic. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a. Electrochemical Cell Explained.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemical Cell Explained Know the difference between galvanic. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In this article, we will learn about the basic concepts and topics of electrochemical. Electrochemical Cell Explained.
From learningkurugon1.z22.web.core.windows.net
Electrochemical Cells Gcse Chemistry Electrochemical Cell Explained An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Describe the basic components of electrochemical cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to. Electrochemical Cell Explained.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Electrochemical Cell Explained An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. An electrochemical cell is a device that produces an electric current from energy released by. Electrochemical Cell Explained.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrochemical Cell Explained An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. This kind of cell includes the galvanic, or voltaic, cell, named after. Know the difference between galvanic. An electrochemical cell is a device. Electrochemical Cell Explained.
From greenenergymaterial.com
Basics Of Electrochemical Cells » Green Energy Material Electrochemical Cell Explained An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. Describe. Electrochemical Cell Explained.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Electrochemical Cell Explained In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. Describe the basic components of electrochemical cells. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that. Electrochemical Cell Explained.
From saylordotorg.github.io
Electrochemistry Electrochemical Cell Explained An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Describe the basic components of electrochemical cells.. Electrochemical Cell Explained.
From www.youtube.com
what is electrochemical cell explainclass 11th chemistry redox Electrochemical Cell Explained This kind of cell includes the galvanic, or voltaic, cell, named after. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. Describe the basic components of electrochemical cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to. Electrochemical Cell Explained.
From mavink.com
Diagram Of Electrochemical Cell Electrochemical Cell Explained An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. List some of the characteristics, applications and limitations of cells. Electrochemical Cell Explained.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrochemical Cell Explained An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of cell includes the galvanic, or voltaic, cell, named after. Describe the basic components of electrochemical cells. Know the difference between galvanic. An apparatus. Electrochemical Cell Explained.
From www.pearson.com
Electrochemical Cells Video Tutorial & Practice Channels for Pearson+ Electrochemical Cell Explained An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Describe the basic components of electrochemical cells. Know the difference between galvanic. An electrochemical cell splits the oxidant and. Electrochemical Cell Explained.
From www.coursehero.com
[Solved] 1. The diagram shows an electrochemical cell with copper Electrochemical Cell Explained An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Describe the basic components of electrochemical cells. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. An electrochemical cell splits the oxidant and reductant in a manner that. Electrochemical Cell Explained.
From www.researchgate.net
Classification of electrochemical cells [26] Download Scientific Diagram Electrochemical Cell Explained In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Describe. Electrochemical Cell Explained.
From www.coursehero.com
[Solved] 1. The diagram shows an electrochemical cell with copper Electrochemical Cell Explained In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. Describe the basic components of electrochemical cells. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An electrochemical cell is an apparatus or device that produces electric current from chemical change and. Electrochemical Cell Explained.
From mmerevise.co.uk
Electrochemical Cells MME Electrochemical Cell Explained In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. Know the difference between galvanic. List some of the characteristics, applications and limitations of cells and batteries. Describe the basic components of electrochemical cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or,. Electrochemical Cell Explained.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrochemical Cell Explained An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Know the difference between galvanic. An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. This kind of cell includes the galvanic, or voltaic, cell, named after. List some of the characteristics,. Electrochemical Cell Explained.
From studylib.net
Electrochemical cells Electrochemical Cell Explained Know the difference between galvanic. An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. Describe the basic components of electrochemical cells. An electrochemical cell. Electrochemical Cell Explained.
From in.pinterest.com
What is Electrochemical Cell Notation Line notation Cell Diagram Electrochemical Cell Explained List some of the characteristics, applications and limitations of cells and batteries. Know the difference between galvanic. Describe the basic components of electrochemical cells. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. An. Electrochemical Cell Explained.
From www.youtube.com
GCSE CHEMISTRY ELECTRO CHEMISTRY LESSON 10 simple voltaic cell Electrochemical Cell Explained An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. Know the difference between galvanic. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. List some of the characteristics, applications and limitations of cells and batteries. In this article, we will. Electrochemical Cell Explained.
From mavink.com
Electrochemical Cell Diagram Electrochemical Cell Explained In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous. Electrochemical Cell Explained.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 Electrochemical Cell Explained An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. This kind of cell includes the galvanic, or voltaic, cell, named after. In this article, we will learn about the basic concepts and topics of electrochemical. Electrochemical Cell Explained.
From mavink.com
Salt Bridge Electrochemical Cell Electrochemical Cell Explained Know the difference between galvanic. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. List some of the characteristics, applications and limitations of cells and batteries. This kind of cell includes the galvanic, or voltaic, cell, named after. An electrochemical cell is a device that produces an. Electrochemical Cell Explained.
From stoplearn.com
Electrochemical Cells 2023 Electrochemical Cell Explained Describe the basic components of electrochemical cells. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. An electrochemical cell is a device that produces. Electrochemical Cell Explained.
From www.scienceabc.com
(Photo Credit Nandalal Sarkar/Shutterstock) Electrochemical Cell Explained An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. Know the difference between galvanic. An electrochemical cell splits the oxidant and reductant in a manner that allows. Electrochemical Cell Explained.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrochemical Cell Explained An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. Know the difference between galvanic. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. List some of the characteristics, applications and limitations of cells and batteries. An electrochemical cell is an apparatus. Electrochemical Cell Explained.
From www.youtube.com
electrochemical cell electrochemistry class 12 chemistry subject notes Electrochemical Cell Explained An electrochemical cell is an apparatus or device that produces electric current from chemical change and energy released by this spontaneous redox reaction. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely,. Electrochemical Cell Explained.
From mavink.com
Electrochemical Cell Diagram Electrochemical Cell Explained Describe the basic components of electrochemical cells. Know the difference between galvanic. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. An electrochemical cell splits the oxidant and reductant in a manner that allows electrons to. An apparatus that is used to generate electricity from a spontaneous. Electrochemical Cell Explained.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Electrochemical Cell Explained List some of the characteristics, applications and limitations of cells and batteries. In this article, we will learn about the basic concepts and topics of electrochemical cell, its representation, diagram, working principle, structure and. An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. Know the difference between galvanic. An electrochemical. Electrochemical Cell Explained.
From www.coursehero.com
[Solved] The diagram shows an electrochemical cell with a gold strip Electrochemical Cell Explained An electrochemical cell is a device that produces an electric current from energy released by a spontaneous redox reaction. List some of the characteristics, applications and limitations of cells and batteries. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous. Describe the basic components of electrochemical cells.. Electrochemical Cell Explained.