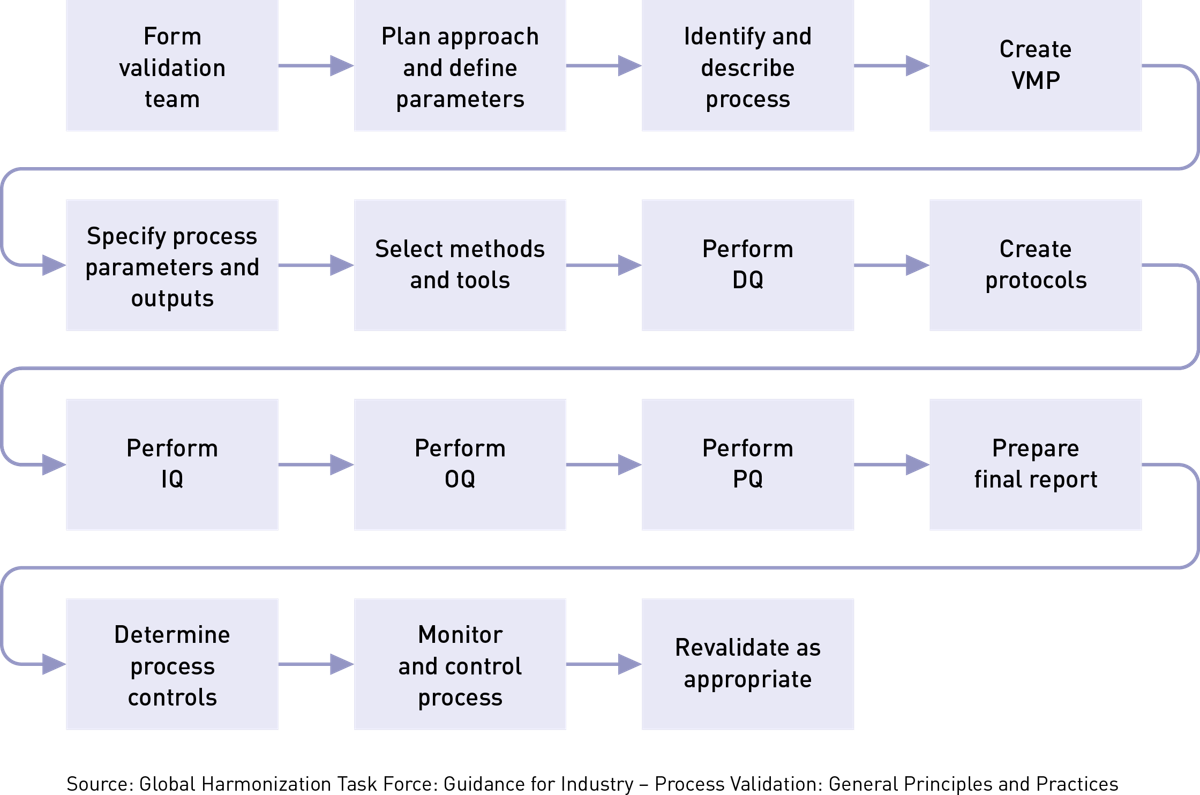
Medical Device Verification And Validation . Food and drug administration (fda) and international regulatory standards continue to evolve and become. Process verification and process validation are required activities for medical device manufacturers who are required. Learn the difference between verification and validation, two essential steps in the medical device development process. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Validation and verification for medical devices. Validation for medical device product quality assurance. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use.
from www.orielstat.com
Food and drug administration (fda) and international regulatory standards continue to evolve and become. Validation and verification for medical devices. Validation for medical device product quality assurance. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Process verification and process validation are required activities for medical device manufacturers who are required. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Learn the difference between verification and validation, two essential steps in the medical device development process.
Medical Device Process Validation What You Need to Know
Medical Device Verification And Validation Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Validation and verification for medical devices. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Process verification and process validation are required activities for medical device manufacturers who are required. Food and drug administration (fda) and international regulatory standards continue to evolve and become. Validation for medical device product quality assurance. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Learn the difference between verification and validation, two essential steps in the medical device development process. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Medical Device Verification And Validation Process verification and process validation are required activities for medical device manufacturers who are required. Learn the difference between verification and validation, two essential steps in the medical device development process. Validation and verification for medical devices. Food and drug administration (fda) and international regulatory standards continue to evolve and become. Often, they happen in combination, with verification occurring during. Medical Device Verification And Validation.
From www.greenlight.guru
Design Verification & Validation for Medical Devices [Guide] Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at. Medical Device Verification And Validation.
From www.scribd.com
Medical Devices Verification and Validation (V&V) Solution Engineering Medical Device Verification And Validation Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Learn the difference between verification and validation, two essential steps in the medical device development process. Process verification and process validation are required activities for medical device manufacturers who are required. Validation and verification for medical devices. Validation. Medical Device Verification And Validation.
From www.orielstat.com
Medical Device Process Validation What You Need to Know Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Validation for medical device product quality assurance. Verification tests whether the design outputs match the inputs, while validation. Medical Device Verification And Validation.
From www.researchandmarkets.com
Understanding FDA Design Verification and Validation Requirements for Medical Device Verification And Validation Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Process verification and process validation are required activities for medical device manufacturers who are required.. Medical Device Verification And Validation.
From bluefruit.co.uk
V&V Testing Signs you have a verification & validation issue Medical Device Verification And Validation Food and drug administration (fda) and international regulatory standards continue to evolve and become. Process verification and process validation are required activities for medical device manufacturers who are required. Validation and verification for medical devices. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use.. Medical Device Verification And Validation.
From 4easyreg.com
Process Validation for Medical Devices Overview of FDA Requirements Medical Device Verification And Validation Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Validation for medical device product quality assurance. Learn the difference between verification and validation, two essential steps in the medical device development process. Validation and verification for medical devices. Often, they happen in combination, with verification occurring during. Medical Device Verification And Validation.
From academy.qualitymeddev.com
Medical Device Software Verification & Validation QualityMedDev Academy Medical Device Verification And Validation Process verification and process validation are required activities for medical device manufacturers who are required. Food and drug administration (fda) and international regulatory standards continue to evolve and become. Validation and verification for medical devices. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Learn the difference between verification. Medical Device Verification And Validation.
From www.qualitymeddev.com
IEC 623042006 Medical Device Software QualityMedDev Medical Device Verification And Validation Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Learn the difference between verification and validation, two essential steps in the medical device development process. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Validation and. Medical Device Verification And Validation.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Medical Device Verification And Validation Food and drug administration (fda) and international regulatory standards continue to evolve and become. Validation and verification for medical devices. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Validation for medical device product quality assurance. Verification and validation are design controls required by the fda to ensure the. Medical Device Verification And Validation.
From www.youtube.com
Difference between Verification and Validation ISO 9001 Definitions Medical Device Verification And Validation Validation for medical device product quality assurance. Food and drug administration (fda) and international regulatory standards continue to evolve and become. Validation and verification for medical devices. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Often, they happen in combination, with verification occurring during the medical device design process and validation. Medical Device Verification And Validation.
From www.orielstat.com
Overview of Medical Device Process Validation IQ, OQ, and PQ Oriel Medical Device Verification And Validation Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Food and drug administration (fda) and international regulatory standards continue to evolve and become. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended. Medical Device Verification And Validation.
From docslib.org
Process Verification & Validation for Medical Devices Using Additive Medical Device Verification And Validation Validation and verification for medical devices. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Food and drug administration (fda) and international regulatory standards continue to evolve and become. Process verification and process validation are required activities for medical device manufacturers who are required. Verification tests whether the design. Medical Device Verification And Validation.
From tsquality.ch
The Future of Validation & Verification in Medical Devices Industry Medical Device Verification And Validation Food and drug administration (fda) and international regulatory standards continue to evolve and become. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Validation and verification for medical devices. Process verification and process validation are required activities for medical device manufacturers who are required. Validation and verification. Medical Device Verification And Validation.
From www.slideteam.net
Medical Device Process Validation Flowchart Medical Device Verification And Validation Validation for medical device product quality assurance. Process verification and process validation are required activities for medical device manufacturers who are required. Learn the difference between verification and validation, two essential steps in the medical device development process. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use.. Medical Device Verification And Validation.
From www.orielstat.com
Medical Device Process Validation Overview & Steps Oriel STAT A MATRIX Medical Device Verification And Validation Validation and verification for medical devices. Food and drug administration (fda) and international regulatory standards continue to evolve and become. Learn the difference between verification and validation, two essential steps in the medical device development process. Validation for medical device product quality assurance. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the. Medical Device Verification And Validation.
From www.presentationeze.com
Medical Device Validation Requirements Principles & Practices Medical Device Verification And Validation Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Validation. Medical Device Verification And Validation.
From www.joharidigital.com
The Ultimate guide to Validation & Verification of Medical Devices Medical Device Verification And Validation Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Learn the difference between verification and validation, two essential steps in the medical device development process. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended. Medical Device Verification And Validation.
From data1.skinnyms.com
Medical Device Verification And Validation Plan Template Medical Device Verification And Validation Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Process verification and process validation are required activities for medical device manufacturers who are required. Validation for medical. Medical Device Verification And Validation.
From www.padtinc.com
FDA Opening to Simulation Supported Verification and Validation for Medical Device Verification And Validation Validation and verification for medical devices. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture. Medical Device Verification And Validation.
From flamlabelthema.netlify.app
Medical Device Test Method Validation Template Medical Device Verification And Validation Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Validation and verification for medical devices. Learn the difference between verification and validation, two essential steps in the medical device development process. Often, they happen in combination, with verification occurring during the medical device design process and validation. Medical Device Verification And Validation.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Verification And Validation Learn the difference between verification and validation, two essential steps in the medical device development process. Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Validation and. Medical Device Verification And Validation.
From industrytechnologyreports.home.blog
Medical Device Validation & Verification Market Worth 1.1 Billion By Medical Device Verification And Validation Food and drug administration (fda) and international regulatory standards continue to evolve and become. Process verification and process validation are required activities for medical device manufacturers who are required. Validation and verification for medical devices. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use.. Medical Device Verification And Validation.
From www.orielstat.com
Medical Device Validation Training Oriel STAT A MATRIX Medical Device Verification And Validation Food and drug administration (fda) and international regulatory standards continue to evolve and become. Learn the difference between verification and validation, two essential steps in the medical device development process. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Verification and validation are design controls required by. Medical Device Verification And Validation.
From medicaldeviceacademy.com
Define medical device software verification and validation (V&V Medical Device Verification And Validation Learn the difference between verification and validation, two essential steps in the medical device development process. Validation for medical device product quality assurance. Validation and verification for medical devices. Process verification and process validation are required activities for medical device manufacturers who are required. Food and drug administration (fda) and international regulatory standards continue to evolve and become. Verification tests. Medical Device Verification And Validation.
From www.emergobyul.com
Analysis of design validation and verification for medical device human Medical Device Verification And Validation Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Validation and verification for medical devices. Learn the difference between verification and validation, two essential steps in the medical device development process. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user. Medical Device Verification And Validation.
From fasttrackiso13485.com
Fast Track ISO 13485 Process Validation Explained for your Medical Device Medical Device Verification And Validation Validation for medical device product quality assurance. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Process verification and process validation are required activities for medical device manufacturers who are required. Validation and verification are inextricable from product development and process design for the manufacturing of medical. Medical Device Verification And Validation.
From www.scribd.com
Medical Device Software Verification, Validation and Compliance (David Medical Device Verification And Validation Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Learn the difference between verification and validation, two essential steps in the medical device development process. Process verification. Medical Device Verification And Validation.
From operonstrategist.com
Guide to Medical Device Process Validation in Manufacturing Operon Medical Device Verification And Validation Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Food and drug administration (fda) and international regulatory standards continue to evolve and become. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Validation for medical device product quality assurance.. Medical Device Verification And Validation.
From www.omnica.com
Medical Device Verification and Validation Omnica Corporation Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Verification tests whether the design outputs match the inputs, while validation tests whether the device. Medical Device Verification And Validation.
From prntbl.concejomunicipaldechinu.gov.co
Medical Device Verification And Validation Plan Template prntbl Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Validation and verification for medical devices. Validation for medical device product quality assurance. Food and drug administration (fda) and international regulatory standards continue to evolve and become. Process verification and process validation are required activities. Medical Device Verification And Validation.
From www.scribd.com
Medical Device Design Validation SOP Verification And Validation Medical Device Verification And Validation Validation and verification are inextricable from product development and process design for the manufacturing of medical devices. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Validation. Medical Device Verification And Validation.
From academycenters.com
Verification & Validation of Medical Devices Medical Device Verification And Validation Validation for medical device product quality assurance. Learn the difference between verification and validation, two essential steps in the medical device development process. Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Validation and verification for medical devices. Process verification and process validation are. Medical Device Verification And Validation.
From templates.rjuuc.edu.np
Medical Device Verification And Validation Plan Template Medical Device Verification And Validation Validation and verification for medical devices. Learn the difference between verification and validation, two essential steps in the medical device development process. Verification tests whether the design outputs match the inputs, while validation tests whether the device meets the user needs and intended use. Process verification and process validation are required activities for medical device manufacturers who are required. Validation. Medical Device Verification And Validation.
From easymedicaldevice.com
Process Validation or Verification (Medical Device)? Medical Device Verification And Validation Verification and validation are design controls required by the fda to ensure the medical devices you manufacture are safe, effective, and fit the intended use. Often, they happen in combination, with verification occurring during the medical device design process and validation happening at the end. Validation and verification are inextricable from product development and process design for the manufacturing of. Medical Device Verification And Validation.