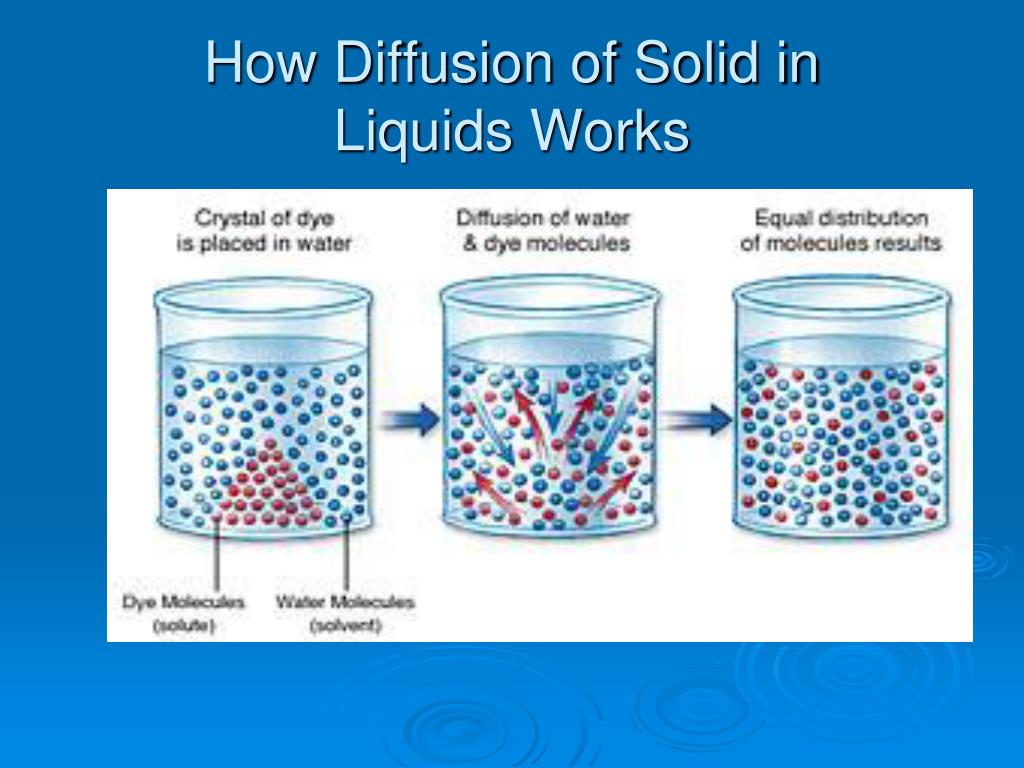
Solid In Water Solution Example . On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. For example, the main solute in sea. This module explains these terms, and sketch out a diagram that. A key to understanding the solubility of ionic solids in water are the concepts of lattice energy and hydration energy. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Many alloys are solid solutions of one metal dissolved in another; An aqueous solution is water that contains one or more dissolved substance. That is, both solute and solvent can be recovered in chemically unchanged forms using. The dissolved substances in an aqueous solution may be. The formation of a solution from a solute and a solvent is a physical process, not a chemical one.
from www.slideserve.com
Many alloys are solid solutions of one metal dissolved in another; A key to understanding the solubility of ionic solids in water are the concepts of lattice energy and hydration energy. An aqueous solution is water that contains one or more dissolved substance. The dissolved substances in an aqueous solution may be. For example, the main solute in sea. This module explains these terms, and sketch out a diagram that. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. That is, both solute and solvent can be recovered in chemically unchanged forms using. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions.
PPT Water and its relation to Diffusion and Osmosis PowerPoint
Solid In Water Solution Example This module explains these terms, and sketch out a diagram that. A key to understanding the solubility of ionic solids in water are the concepts of lattice energy and hydration energy. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. For example, the main solute in sea. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. The dissolved substances in an aqueous solution may be. An aqueous solution is water that contains one or more dissolved substance. That is, both solute and solvent can be recovered in chemically unchanged forms using. Many alloys are solid solutions of one metal dissolved in another; Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. This module explains these terms, and sketch out a diagram that.
From www.corrosionpedia.com
Total Solids Solid In Water Solution Example Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. An aqueous solution is water that contains one or more dissolved substance. The formation of a solution from a solute and a solvent is a. Solid In Water Solution Example.
From www.youtube.com
Total dissolved solids YouTube Solid In Water Solution Example The formation of a solution from a solute and a solvent is a physical process, not a chemical one. The dissolved substances in an aqueous solution may be. Many alloys are solid solutions of one metal dissolved in another; For example, the main solute in sea. That is, both solute and solvent can be recovered in chemically unchanged forms using.. Solid In Water Solution Example.
From www.slideserve.com
PPT Water The Universal Solvent PowerPoint Presentation, free Solid In Water Solution Example The formation of a solution from a solute and a solvent is a physical process, not a chemical one. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. The dissolved substances in an aqueous solution may be. An aqueous solution is water that contains one or more dissolved substance. Solute. Solid In Water Solution Example.
From www.teachoo.com
Diffusion in Solids, Liquids and Gases with Examples Teachoo Solid In Water Solution Example The dissolved substances in an aqueous solution may be. This module explains these terms, and sketch out a diagram that. An aqueous solution is water that contains one or more dissolved substance. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. A key to understanding the solubility of ionic solids. Solid In Water Solution Example.
From quizlet.com
investigate the solubility of a solid in water at a specific Solid In Water Solution Example Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. The dissolved substances in an aqueous solution may be. The formation of a solution from a solute and a solvent is a physical process, not. Solid In Water Solution Example.
From joiicrblp.blob.core.windows.net
What Is Solid Liquid Solution at Nicholas Hartman blog Solid In Water Solution Example The dissolved substances in an aqueous solution may be. That is, both solute and solvent can be recovered in chemically unchanged forms using. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. An aqueous solution is water that contains one or more dissolved substance. Many alloys are solid solutions of. Solid In Water Solution Example.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Solid In Water Solution Example The formation of a solution from a solute and a solvent is a physical process, not a chemical one. The dissolved substances in an aqueous solution may be. An aqueous solution is water that contains one or more dissolved substance. For example, the main solute in sea. That is, both solute and solvent can be recovered in chemically unchanged forms. Solid In Water Solution Example.
From primaryleap.co.uk
Chemistry Solutions And Mixtures Level 1 activity for kids Solid In Water Solution Example Many alloys are solid solutions of one metal dissolved in another; Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. This module explains these terms, and sketch out a diagram that. The dissolved substances in an aqueous solution may be. That is, both solute and solvent can be recovered in chemically unchanged forms. Solid In Water Solution Example.
From sciencenotes.org
What Is an Aqueous Solution? Definition and Examples Solid In Water Solution Example This module explains these terms, and sketch out a diagram that. For example, the main solute in sea. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. Many alloys are solid. Solid In Water Solution Example.
From www.aplustopper.com
How can we Separate a Mixture of a Solid and a Liquid using Evaporation Solid In Water Solution Example A key to understanding the solubility of ionic solids in water are the concepts of lattice energy and hydration energy. For example, the main solute in sea. The dissolved substances in an aqueous solution may be. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Solute the solid (or occasionally. Solid In Water Solution Example.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize Solid In Water Solution Example Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. This module explains these terms, and sketch out a diagram that. Many alloys are solid solutions of one metal dissolved in another; Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. For example, the. Solid In Water Solution Example.
From www.kidpid.com
States of Water Kidpid Solid In Water Solution Example An aqueous solution is water that contains one or more dissolved substance. For example, the main solute in sea. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. That is, both solute and solvent can be recovered in chemically unchanged forms using. Solute the solid (or occasionally a gas) which dissolves into a. Solid In Water Solution Example.
From www.wikidoc.org
Solution wikidoc Solid In Water Solution Example Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Many alloys are solid solutions of one metal dissolved in another; The dissolved substances in an aqueous solution may be. An aqueous solution is water. Solid In Water Solution Example.
From www.freepik.com
Free Vector Dissolving science experiment for kids Solid In Water Solution Example This module explains these terms, and sketch out a diagram that. That is, both solute and solvent can be recovered in chemically unchanged forms using. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. The dissolved substances in an aqueous solution may be. A key to understanding the solubility of ionic solids in. Solid In Water Solution Example.
From www.slideserve.com
PPT Solute and Solvent PowerPoint Presentation, free download ID Solid In Water Solution Example A key to understanding the solubility of ionic solids in water are the concepts of lattice energy and hydration energy. This module explains these terms, and sketch out a diagram that. That is, both solute and solvent can be recovered in chemically unchanged forms using. An aqueous solution is water that contains one or more dissolved substance. Solid solutions are. Solid In Water Solution Example.
From www.teachoo.com
Solution Definition, Types, Properties Chemistry Teachoo Solid In Water Solution Example Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. An aqueous solution is water that contains one or more dissolved substance. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. This module explains these terms, and sketch out a diagram that. Many alloys. Solid In Water Solution Example.
From www.twinkl.co.cr
What is Dissolving? Answered Twinkl Teaching Wiki Solid In Water Solution Example This module explains these terms, and sketch out a diagram that. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in. Solid In Water Solution Example.
From www.youtube.com
Solubility of solids in liquids YouTube Solid In Water Solution Example Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. For example, the main solute in sea. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. An aqueous solution is water that contains one or more dissolved substance. The formation of a solution from. Solid In Water Solution Example.
From www.snexplores.org
Explainer What are the different states of matter? Solid In Water Solution Example Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. An aqueous solution is water that contains one or more dissolved substance. For example, the main solute in sea. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Solute the solid (or occasionally a. Solid In Water Solution Example.
From www.blendspace.com
Chapter 14 Aqueous Solutions Lessons Blendspace Solid In Water Solution Example The formation of a solution from a solute and a solvent is a physical process, not a chemical one. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. A key to understanding the solubility of ionic solids in water are the concepts of lattice energy and hydration energy. Solid solutions. Solid In Water Solution Example.
From www.alamy.com
Water States of matter Phase. Change of State for Water Diagram Solid In Water Solution Example The formation of a solution from a solute and a solvent is a physical process, not a chemical one. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. That is, both solute and solvent can be recovered in chemically unchanged forms using. This module explains these terms, and sketch out. Solid In Water Solution Example.
From sciencemsqblog8.blogspot.com
Science8 Semester 2,Chapter 4 Mixtures Solid In Water Solution Example Many alloys are solid solutions of one metal dissolved in another; On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. The dissolved substances in an aqueous solution may be. For example, the main solute in sea. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in. Solid In Water Solution Example.
From webmis.highland.cc.il.us
Aqueous Solutions Solid In Water Solution Example For example, the main solute in sea. The dissolved substances in an aqueous solution may be. This module explains these terms, and sketch out a diagram that. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. Many alloys are solid solutions of one metal dissolved in another; That is, both solute and solvent. Solid In Water Solution Example.
From www.science-sparks.com
Which solids dissolve in water Solid In Water Solution Example A key to understanding the solubility of ionic solids in water are the concepts of lattice energy and hydration energy. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. That is,. Solid In Water Solution Example.
From educatorpages.com
Chemistry Solid In Water Solution Example An aqueous solution is water that contains one or more dissolved substance. This module explains these terms, and sketch out a diagram that. The dissolved substances in an aqueous solution may be. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. That is, both solute and solvent can be recovered. Solid In Water Solution Example.
From www.slideshare.net
Water and solutions Solid In Water Solution Example Many alloys are solid solutions of one metal dissolved in another; The dissolved substances in an aqueous solution may be. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. That is, both solute and solvent can be recovered in chemically unchanged forms using. The formation of a solution from a. Solid In Water Solution Example.
From www.bbc.com
What is water? BBC Bitesize Solid In Water Solution Example Many alloys are solid solutions of one metal dissolved in another; That is, both solute and solvent can be recovered in chemically unchanged forms using. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. The formation of a solution from a solute and a solvent is a physical process, not. Solid In Water Solution Example.
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Solid In Water Solution Example Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. Many alloys are solid solutions of one metal dissolved in another; This module explains these terms, and sketch out a diagram that. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. For example, the. Solid In Water Solution Example.
From www.alamy.com
Density of matter with gas, liquid and solid water states outline Solid In Water Solution Example On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. That is, both solute and solvent can be recovered in chemically unchanged forms using. The dissolved substances in an aqueous solution may be. An aqueous solution is water that contains one or more dissolved substance. A key to understanding the solubility. Solid In Water Solution Example.
From www.slideshare.net
Water and solutions Solid In Water Solution Example On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. That is, both. Solid In Water Solution Example.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Solid In Water Solution Example For example, the main solute in sea. An aqueous solution is water that contains one or more dissolved substance. That is, both solute and solvent can be recovered in chemically unchanged forms using. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. This module explains these terms, and sketch out a diagram that.. Solid In Water Solution Example.
From wou.edu
CH104 Chapter 7 Solutions Chemistry Solid In Water Solution Example The dissolved substances in an aqueous solution may be. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. Many alloys are solid solutions of one metal dissolved in another; Solute the solid (or occasionally a gas) which dissolves into a solvent (liquid) in order to make a solution. This module. Solid In Water Solution Example.
From www.slideserve.com
PPT Water and its relation to Diffusion and Osmosis PowerPoint Solid In Water Solution Example The formation of a solution from a solute and a solvent is a physical process, not a chemical one. Solid solutions are a heterogeneous mixture in which solute, or dispersed substance, and solvent, or dispersed. The dissolved substances in an aqueous solution may be. For example, the main solute in sea. Many alloys are solid solutions of one metal dissolved. Solid In Water Solution Example.
From hive.blog
Determination of total solids in water samples — Hive Solid In Water Solution Example A key to understanding the solubility of ionic solids in water are the concepts of lattice energy and hydration energy. That is, both solute and solvent can be recovered in chemically unchanged forms using. The dissolved substances in an aqueous solution may be. For example, the main solute in sea. The formation of a solution from a solute and a. Solid In Water Solution Example.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solid In Water Solution Example This module explains these terms, and sketch out a diagram that. The dissolved substances in an aqueous solution may be. On the basis of physical states of solvent and solute can be categorized as solid, liquid and gaseous solutions. The formation of a solution from a solute and a solvent is a physical process, not a chemical one. That is,. Solid In Water Solution Example.