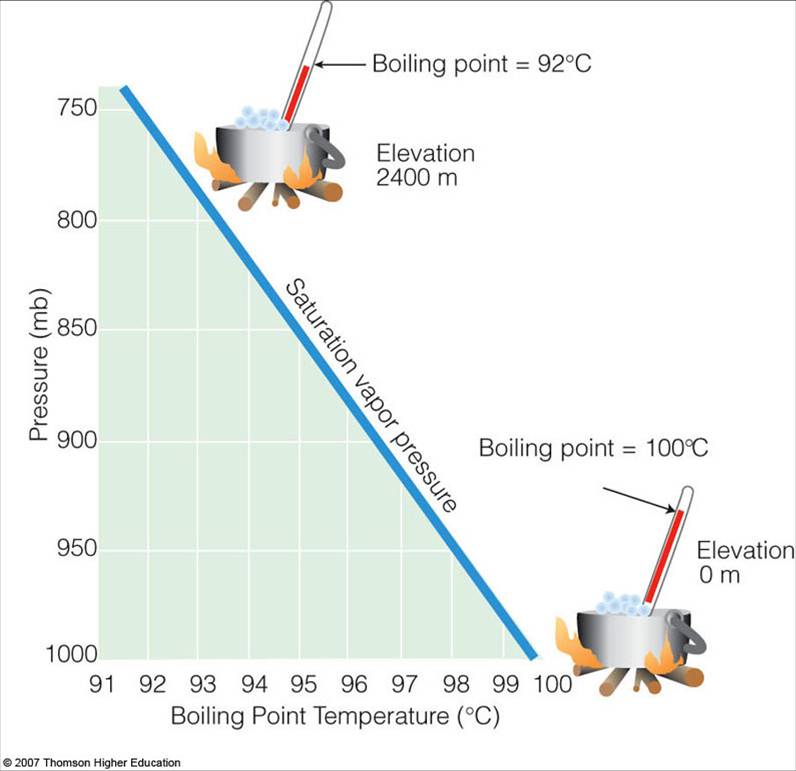
Does Water Boil Pressure . 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. The boiling point of water also depends on the water's purity. The boiling point at different levels of. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. What causes water to boil is. However, at higher altitudes, hence lower. at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure.
from apollo.lsc.vsc.edu
online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). What causes water to boil is. The boiling point at different levels of. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. However, at higher altitudes, hence lower.
Saturation Vapor Pressure and the Boiling Point
Does Water Boil Pressure What causes water to boil is. water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. The boiling point at different levels of. at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. What causes water to boil is. The boiling point of water also depends on the water's purity. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). However, at higher altitudes, hence lower. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure.
From aweseas.blogspot.com
Boiling Point Of Water Sea Level Does Water Boil Pressure As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. The boiling point at different levels of. at 23°c, for example, water would boil at a. Does Water Boil Pressure.
From www.pinterest.com
Mastering Pressure Cooking Understanding Boiling Point at Different Does Water Boil Pressure However, at higher altitudes, hence lower. What causes water to boil is. water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric pressure (coming back down. Does Water Boil Pressure.
From sciencenotes.org
How to Boil Water at Room Temperature Does Water Boil Pressure The boiling point at different levels of. However, at higher altitudes, hence lower. online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. at 23°c, for example,. Does Water Boil Pressure.
From mungfali.com
Water Boiling Point Pressure Chart Does Water Boil Pressure online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). The boiling point of water also depends on the water's purity. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase. Does Water Boil Pressure.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does Water Boil Pressure The boiling point at different levels of. What causes water to boil is. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature. Does Water Boil Pressure.
From www.youtube.com
Boiling point and external pressure YouTube Does Water Boil Pressure The boiling point at different levels of. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level. Does Water Boil Pressure.
From gioumbril.blob.core.windows.net
Does Water Boil At Lower Pressure at Elizabeth Jessen blog Does Water Boil Pressure What causes water to boil is. However, at higher altitudes, hence lower. at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. online calculator, figures. Does Water Boil Pressure.
From hxeixhnxc.blob.core.windows.net
Boil The Water Meaning at Barbara Fitchett blog Does Water Boil Pressure water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water. Does Water Boil Pressure.
From mavink.com
Water Boiling Point Pressure Chart Does Water Boil Pressure at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. at sea level, vapour pressure is equal to the atmospheric pressure. Does Water Boil Pressure.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point Does Water Boil Pressure at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. The boiling point of water also depends on the water's purity. The boiling point at different levels of. However, at higher altitudes, hence lower. at sea level, vapour pressure is equal to the atmospheric pressure at 100. Does Water Boil Pressure.
From mavink.com
Water Boiling Point Vs Pressure Chart Does Water Boil Pressure 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. The boiling point of water also depends on the water's purity. online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). at sea level, vapour pressure is. Does Water Boil Pressure.
From mavink.com
Water Boiling Pressure Chart Does Water Boil Pressure As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. The boiling point of water also depends on the water's purity. at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. . Does Water Boil Pressure.
From www.youtube.com
Why does water's boiling point change at different levels of pressure Does Water Boil Pressure What causes water to boil is. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and. Does Water Boil Pressure.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point Does Water Boil Pressure As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. However, at higher altitudes, hence lower. water boils. Does Water Boil Pressure.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does Water Boil Pressure As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. However, at higher altitudes, hence lower. at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. water boils. Does Water Boil Pressure.
From www.slideserve.com
PPT Ch15 Global Circulation and Weather PowerPoint Presentation ID Does Water Boil Pressure water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. However, at higher altitudes, hence lower. What causes water to boil is. The boiling point of water also depends on the. Does Water Boil Pressure.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point Does Water Boil Pressure What causes water to boil is. The boiling point at different levels of. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). The boiling point of water also depends on the. Does Water Boil Pressure.
From slideplayer.com
Latent Heat Can water boil at temperatures other than 100 oC? ppt Does Water Boil Pressure The boiling point of water also depends on the water's purity. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). What causes water to boil is. 95 rows when water. Does Water Boil Pressure.
From www.tec-science.com
Why does water boil faster at high altitudes? tecscience Does Water Boil Pressure at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. The boiling point of water also depends on the water's purity. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric. Does Water Boil Pressure.
From labbyag.es
Water Boiling Temperature Pressure Chart Labb by AG Does Water Boil Pressure 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). . Does Water Boil Pressure.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of Does Water Boil Pressure at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. The boiling point at different levels of. However, at higher altitudes, hence lower. water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. water boils at a lower temperature as. Does Water Boil Pressure.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Does Water Boil Pressure water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). The boiling point of water also depends on the water's purity. 95 rows when water is heated up under pressure, its. Does Water Boil Pressure.
From foodandfizz.com
Does Water Boil Faster With A Lid? 1 Best Answer Does Water Boil Pressure 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). The boiling point. Does Water Boil Pressure.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Does Water Boil Pressure 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. What causes water to boil is. water boils at a lower temperature as you gain. Does Water Boil Pressure.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Does Water Boil Pressure The boiling point at different levels of. at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). However, at higher altitudes, hence lower. 95. Does Water Boil Pressure.
From mavink.com
Water Boiling Point Pressure Chart Does Water Boil Pressure water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. The boiling point of water also depends on the water's purity. at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. As we move higher into the atmosphere and the atmospheric. Does Water Boil Pressure.
From www.slideserve.com
PPT Ch. 2 States of Matter PowerPoint Presentation, free download Does Water Boil Pressure The boiling point of water also depends on the water's purity. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. at sea level,. Does Water Boil Pressure.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation Does Water Boil Pressure at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric. Does Water Boil Pressure.
From giojjcdzd.blob.core.windows.net
What Happens To The Water When It Boils at Elaine Morin blog Does Water Boil Pressure water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if you increase atmospheric pressure (coming back down to sea level or going below it). at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure.. Does Water Boil Pressure.
From aweseas.blogspot.com
Boiling Point Of Water At Sea Level In Kelvin Does Water Boil Pressure online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). The boiling point at different levels of. at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. As we move higher into the atmosphere and. Does Water Boil Pressure.
From mungfali.com
Water Boiling Point Pressure Chart Does Water Boil Pressure As we move higher into the atmosphere and the atmospheric pressure drops, so too does the amount of vapour pressure required for a liquid to boil. at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. water boils at 100 °c (212 °f) at. Does Water Boil Pressure.
From www.youtube.com
Does Water Really Boil in a Vacuum Chamber? And Why? YouTube Does Water Boil Pressure online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). The boiling point at different levels of. What causes water to boil is. However, at higher altitudes, hence lower. water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. . Does Water Boil Pressure.
From gioumbril.blob.core.windows.net
Does Water Boil At Lower Pressure at Elizabeth Jessen blog Does Water Boil Pressure 95 rows when water is heated up under pressure, its boiling point is higher than at atmospheric pressure. at sea level, vapour pressure is equal to the atmospheric pressure at 100 ˚c, and so this is the temperature at which water boils. The boiling point at different levels of. However, at higher altitudes, hence lower. at 23°c,. Does Water Boil Pressure.
From www.youtube.com
Vapor Pressure and Boiling YouTube Does Water Boil Pressure at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. The boiling point of water also depends on the water's purity. However, at higher altitudes, hence lower. water boils at 100 °c (212 °f) at sea level (0 m), where pressure is higher. online calculator, figures. Does Water Boil Pressure.
From www.kentchemistry.com
Determine Boiling Point from Vapor Pressure Does Water Boil Pressure at 23°c, for example, water would boil at a pressure of about 21.1 torr, or about a fortieth of atmospheric pressure. online calculator, figures and tables showing boiling points of water at pressures ranging from 14.7 to 3200 psia (1 to 220 bara). water boils at 100 °c (212 °f) at sea level (0 m), where pressure. Does Water Boil Pressure.