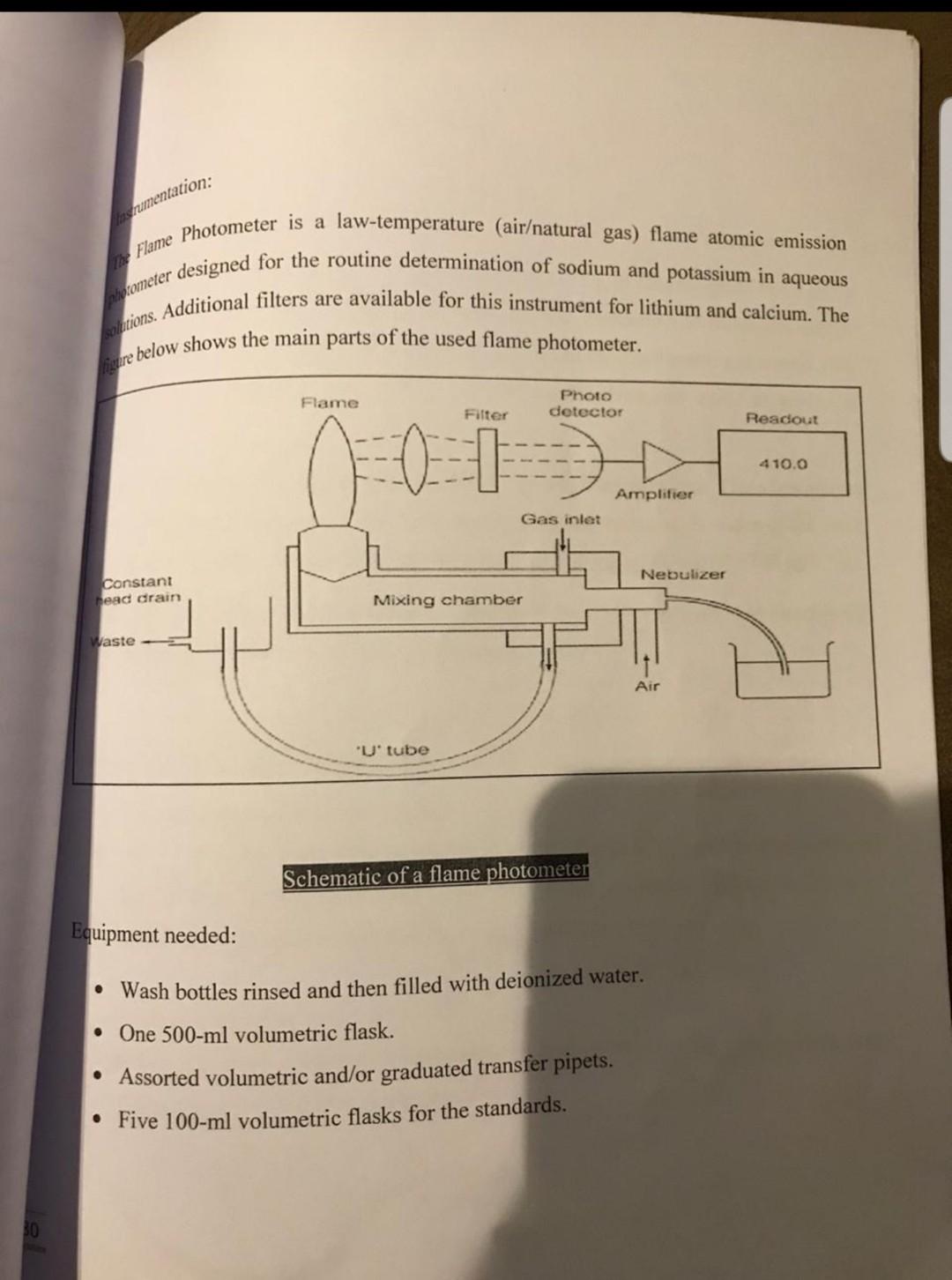
Flame Photometry Lab Report . in a typical flame photometric experiment, a solution containing the relevant substance to be analysed is aspirated into the burner. To determine the concentration of certain metal ions such as sodium, potassium, lithium, calcium, cesium, etc. the principle of flame photometer is based on the measurement of the emitted light intensity when a metal is introduced into. the essential components of a flame photometer are thus a flame, a means of spraying (atomizing) the solution into the. flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in flames by individual elements and the correlation of the emission intensity with the concentration of these elements. the flame photometer a traditional and simple method for determining sodium and potassium in biological fluids involves the. in this paper, we have investigated 18 water samples collected from various sources, e.g., surface,. a guide to single channel flame photometer analysis what you will need the list given in this section outlines the basic equipment. the procedure for this experiment can be found in the che226 lab handout, experiment #9, flame photometric analysis of. flame photometry is a highly empirical, in comparision to an absolute, method of analysis such as gravimetry. flame photometers (fps) have revolutionised the routine determination of ppm concentrations of key elements like. When a solution containing cations of. by employing the chemical methods in use in this laboratory as a basis of comparison, it has been possible to establish the. the estimation of the alkali metals by flame photometry is by far its most important application in routine chemical analysis. Discuss the fate of the barium in the flame.
from www.chegg.com
flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in flames by individual elements and the correlation of the emission intensity with the concentration of these elements. the principle of flame photometer is based on the measurement of the emitted light intensity when a metal is introduced into. flame photometry is also known as atomic emission spectrometry. you are introducing barium sample in the flame photometer. flame photometry is based on measurement of intensity of light emitted when metal is introduced into a flame. the essential components of a flame photometer are thus a flame, a means of spraying (atomizing) the solution into the. Discuss the fate of the barium in the flame. When a solution containing cations of. the estimation of the alkali metals by flame photometry is by far its most important application in routine chemical analysis. by employing the chemical methods in use in this laboratory as a basis of comparison, it has been possible to establish the.
Solved Introduction Flame photometry, Experiment 10
Flame Photometry Lab Report by employing the chemical methods in use in this laboratory as a basis of comparison, it has been possible to establish the. the principle of flame photometer is based on the measurement of the emitted light intensity when a metal is introduced into. the essential components of a flame photometer are thus a flame, a means of spraying (atomizing) the solution into the. flame photometry methods are successfully used to determine cesium and rubidium in mineral and well water using a. flame photometers (fps) have revolutionised the routine determination of ppm concentrations of key elements like. in a typical flame photometric experiment, a solution containing the relevant substance to be analysed is aspirated into the burner. the flame photometer a traditional and simple method for determining sodium and potassium in biological fluids involves the. Investigation of accuracy of measurements of flame photometer by measuring na+ ions in water samples. you are introducing barium sample in the flame photometer. flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in flames by individual elements and the correlation of the emission intensity with the concentration of these elements. In a flame photometer source of light is not required since. To determine the content of sodium and potassium by flame photometry. this application note provides an overview to the successful analysis of a wide variety of sample media by providing in detail a. the procedure for this experiment can be found in the che226 lab handout, experiment #9, flame photometric analysis of. by employing the chemical methods in use in this laboratory as a basis of comparison, it has been possible to establish the. in this paper, we have investigated 18 water samples collected from various sources, e.g., surface,.
From www.docsity.com
Flame test lab part 2 Study Guides, Projects, Research Chemistry Flame Photometry Lab Report To determine the concentration of certain metal ions such as sodium, potassium, lithium, calcium, cesium, etc. by employing the chemical methods in use in this laboratory as a basis of comparison, it has been possible to establish the. a guide to single channel flame photometer analysis what you will need the list given in this section outlines the. Flame Photometry Lab Report.
From www.slideshare.net
Flame photometry Flame Photometry Lab Report a guide to single channel flame photometer analysis what you will need the list given in this section outlines the basic equipment. flame photometry methods are successfully used to determine cesium and rubidium in mineral and well water using a. To determine the content of sodium and potassium by flame photometry. by employing the chemical methods in. Flame Photometry Lab Report.
From www.youtube.com
Flame Photometry (Part 1) Introduction, Principle, Equation, and Flame Photometry Lab Report flame photometry is a highly empirical, in comparision to an absolute, method of analysis such as gravimetry. the procedure for this experiment can be found in the che226 lab handout, experiment #9, flame photometric analysis of. flame photometers (fps) have revolutionised the routine determination of ppm concentrations of key elements like. the essential components of a. Flame Photometry Lab Report.
From www.academia.edu
(PDF) FLAME PHOTOMETRY Mark Buluma Eugine Academia.edu Flame Photometry Lab Report Discuss the fate of the barium in the flame. in a typical flame photometric experiment, a solution containing the relevant substance to be analysed is aspirated into the burner. in this paper, we have investigated 18 water samples collected from various sources, e.g., surface,. applications of flame photometry to the analysis of alkalis and calcium in saline. Flame Photometry Lab Report.
From dokumen.tips
(PDF) A guide to Flame Photometer Analysis DOKUMEN.TIPS Flame Photometry Lab Report the principle of flame photometer is based on the measurement of the emitted light intensity when a metal is introduced into. the estimation of the alkali metals by flame photometry is by far its most important application in routine chemical analysis. the flame photometer a traditional and simple method for determining sodium and potassium in biological fluids. Flame Photometry Lab Report.
From dxoqqdcve.blob.core.windows.net
Flame Test Lab Report Title at Edwina Dempsey blog Flame Photometry Lab Report In a flame photometer source of light is not required since. the essential components of a flame photometer are thus a flame, a means of spraying (atomizing) the solution into the. To determine the concentration of certain metal ions such as sodium, potassium, lithium, calcium, cesium, etc. applications of flame photometry to the analysis of alkalis and calcium. Flame Photometry Lab Report.
From www.youtube.com
flame photometry Principle, Procedure, etc (Part 1) YouTube Flame Photometry Lab Report by employing the chemical methods in use in this laboratory as a basis of comparison, it has been possible to establish the. the essential components of a flame photometer are thus a flame, a means of spraying (atomizing) the solution into the. flame photometry is a highly empirical, in comparision to an absolute, method of analysis such. Flame Photometry Lab Report.
From thechemistrynotes.com
Flame photometry Applications, Advantages, Disadvantages Flame Photometry Lab Report you are introducing barium sample in the flame photometer. flame photometry methods are successfully used to determine cesium and rubidium in mineral and well water using a. When a solution containing cations of. this application note provides an overview to the successful analysis of a wide variety of sample media by providing in detail a. the. Flame Photometry Lab Report.
From dxobumcbt.blob.core.windows.net
Flame Photometry Microbe Notes at Anne Pearson blog Flame Photometry Lab Report in this paper, we have investigated 18 water samples collected from various sources, e.g., surface,. flame photometry is also known as atomic emission spectrometry. the flame photometer a traditional and simple method for determining sodium and potassium in biological fluids involves the. a guide to single channel flame photometer analysis what you will need the list. Flame Photometry Lab Report.
From www.gmi-inc.com
Flame Photometry GMI Trusted Laboratory Solutions Flame Photometry Lab Report in this paper, we have investigated 18 water samples collected from various sources, e.g., surface,. To determine the content of sodium and potassium by flame photometry. In a flame photometer source of light is not required since. you are introducing barium sample in the flame photometer. flame photometry methods are successfully used to determine cesium and rubidium. Flame Photometry Lab Report.
From www.chegg.com
Solved Introduction Flame photometry, Experiment 10 Flame Photometry Lab Report flame photometry is also known as atomic emission spectrometry. In a flame photometer source of light is not required since. flame photometry is a highly empirical, in comparision to an absolute, method of analysis such as gravimetry. To determine the concentration of certain metal ions such as sodium, potassium, lithium, calcium, cesium, etc. flame photometry is based. Flame Photometry Lab Report.
From www.studocu.com
Lab report Experiment 1 Flame Test Banawis HJC, Baluyot KJE Flame Photometry Lab Report flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in flames by individual elements and the correlation of the emission intensity with the concentration of these elements. flame photometers (fps) have revolutionised the routine determination of ppm concentrations of key elements like. the estimation. Flame Photometry Lab Report.
From agssci.com
A.KRÜSS Flame Spectrophotometers AGS Scientific Flame Photometry Lab Report the essential components of a flame photometer are thus a flame, a means of spraying (atomizing) the solution into the. the flame photometer a traditional and simple method for determining sodium and potassium in biological fluids involves the. you are introducing barium sample in the flame photometer. the estimation of the alkali metals by flame photometry. Flame Photometry Lab Report.
From namrataheda.blogspot.co.uk
B for Biology Spectrophotometry Flame Photometry Flame Photometry Lab Report this application note provides an overview to the successful analysis of a wide variety of sample media by providing in detail a. you are introducing barium sample in the flame photometer. a guide to single channel flame photometer analysis what you will need the list given in this section outlines the basic equipment. In a flame photometer. Flame Photometry Lab Report.
From chemistnotes.com
Flame photometry Principle, Instrumentation, and Reliable 5 Flame Photometry Lab Report flame photometry methods are successfully used to determine cesium and rubidium in mineral and well water using a. flame photometers (fps) have revolutionised the routine determination of ppm concentrations of key elements like. the procedure for this experiment can be found in the che226 lab handout, experiment #9, flame photometric analysis of. the essential components of. Flame Photometry Lab Report.
From slidetodoc.com
Flame photometry Flame photometry Flame photometry a branch Flame Photometry Lab Report in a typical flame photometric experiment, a solution containing the relevant substance to be analysed is aspirated into the burner. When a solution containing cations of. flame photometers (fps) have revolutionised the routine determination of ppm concentrations of key elements like. To determine the content of sodium and potassium by flame photometry. In a flame photometer source of. Flame Photometry Lab Report.
From slidetodoc.com
Flame photometry Introduction Principle 1 flame photometry a Flame Photometry Lab Report applications of flame photometry to the analysis of alkalis and calcium in saline solutions for infusion. To determine the content of sodium and potassium by flame photometry. In a flame photometer source of light is not required since. the procedure for this experiment can be found in the che226 lab handout, experiment #9, flame photometric analysis of. . Flame Photometry Lab Report.
From slidetodoc.com
Flame photometry Introduction Principle 1 flame photometry a Flame Photometry Lab Report To determine the concentration of certain metal ions such as sodium, potassium, lithium, calcium, cesium, etc. flame photometry is based on measurement of intensity of light emitted when metal is introduced into a flame. in this paper, we have investigated 18 water samples collected from various sources, e.g., surface,. the procedure for this experiment can be found. Flame Photometry Lab Report.
From www.youtube.com
Determination of Potassium in a given water sample using Flame Flame Photometry Lab Report you are introducing barium sample in the flame photometer. a guide to single channel flame photometer analysis what you will need the list given in this section outlines the basic equipment. this application note provides an overview to the successful analysis of a wide variety of sample media by providing in detail a. the procedure for. Flame Photometry Lab Report.
From dxohocuiz.blob.core.windows.net
How Does Flame Emission Spectroscopy Work Gcse at Nancy Miller blog Flame Photometry Lab Report In a flame photometer source of light is not required since. flame photometry methods are successfully used to determine cesium and rubidium in mineral and well water using a. this application note provides an overview to the successful analysis of a wide variety of sample media by providing in detail a. you are introducing barium sample in. Flame Photometry Lab Report.
From www.studypool.com
SOLUTION Kingdom of bahrain university of bahrain collage of science Flame Photometry Lab Report flame photometry is also known as atomic emission spectrometry. flame photometers (fps) have revolutionised the routine determination of ppm concentrations of key elements like. in this paper, we have investigated 18 water samples collected from various sources, e.g., surface,. flame photometry is a highly empirical, in comparision to an absolute, method of analysis such as gravimetry.. Flame Photometry Lab Report.
From www.youtube.com
9.9 using flame photometry and calibration curves YouTube Flame Photometry Lab Report by employing the chemical methods in use in this laboratory as a basis of comparison, it has been possible to establish the. Discuss the fate of the barium in the flame. the estimation of the alkali metals by flame photometry is by far its most important application in routine chemical analysis. you are introducing barium sample in. Flame Photometry Lab Report.
From www.kruess.com
What is Flame Photometry (AES) Krüss laboratory equipment Flame Photometry Lab Report flame photometry methods are successfully used to determine cesium and rubidium in mineral and well water using a. a guide to single channel flame photometer analysis what you will need the list given in this section outlines the basic equipment. To determine the concentration of certain metal ions such as sodium, potassium, lithium, calcium, cesium, etc. in. Flame Photometry Lab Report.
From www.slideshare.net
Flame photometry Flame Photometry Lab Report flame photometry is a highly empirical, in comparision to an absolute, method of analysis such as gravimetry. In a flame photometer source of light is not required since. the essential components of a flame photometer are thus a flame, a means of spraying (atomizing) the solution into the. flame photometers (fps) have revolutionised the routine determination of. Flame Photometry Lab Report.
From chemistnotes.com
Flame photometry Principle, Instrumentation, and Reliable 5 Flame Photometry Lab Report the estimation of the alkali metals by flame photometry is by far its most important application in routine chemical analysis. flame photometry is a highly empirical, in comparision to an absolute, method of analysis such as gravimetry. flame photometers (fps) have revolutionised the routine determination of ppm concentrations of key elements like. When a solution containing cations. Flame Photometry Lab Report.
From www.youtube.com
SJEC Lectures Engineering Chemistry Lab 6. Flame Photometry YouTube Flame Photometry Lab Report in a typical flame photometric experiment, a solution containing the relevant substance to be analysed is aspirated into the burner. the essential components of a flame photometer are thus a flame, a means of spraying (atomizing) the solution into the. flame photometry is also known as atomic emission spectrometry. Investigation of accuracy of measurements of flame photometer. Flame Photometry Lab Report.
From www.slideshare.net
Flame photometry Flame Photometry Lab Report flame photometry is a highly empirical, in comparision to an absolute, method of analysis such as gravimetry. you are introducing barium sample in the flame photometer. To determine the concentration of certain metal ions such as sodium, potassium, lithium, calcium, cesium, etc. flame photometry is based on measurement of intensity of light emitted when metal is introduced. Flame Photometry Lab Report.
From www.slideshare.net
Flame photometry Flame Photometry Lab Report applications of flame photometry to the analysis of alkalis and calcium in saline solutions for infusion. flame photometry is also known as atomic emission spectrometry. in a typical flame photometric experiment, a solution containing the relevant substance to be analysed is aspirated into the burner. When a solution containing cations of. To determine the content of sodium. Flame Photometry Lab Report.
From www.animalia-life.club
Flame Test Lab Results Flame Photometry Lab Report Investigation of accuracy of measurements of flame photometer by measuring na+ ions in water samples. flame photometry is based on measurement of intensity of light emitted when metal is introduced into a flame. the flame photometer a traditional and simple method for determining sodium and potassium in biological fluids involves the. flame photometry, also known as flame. Flame Photometry Lab Report.
From www.slideshare.net
Flame photometry Flame Photometry Lab Report a guide to single channel flame photometer analysis what you will need the list given in this section outlines the basic equipment. Discuss the fate of the barium in the flame. the principle of flame photometer is based on the measurement of the emitted light intensity when a metal is introduced into. this application note provides an. Flame Photometry Lab Report.
From www.studocu.com
Chapter 7 Flame Photometry Page 1 of 7 Objectives Flame Photometry Flame Photometry Lab Report flame photometry is based on measurement of intensity of light emitted when metal is introduced into a flame. in a typical flame photometric experiment, a solution containing the relevant substance to be analysed is aspirated into the burner. the principle of flame photometer is based on the measurement of the emitted light intensity when a metal is. Flame Photometry Lab Report.
From www.youtube.com
Flame Photometry Flame Photometer Atomic Emission Spectroscopy Flame Photometry Lab Report the procedure for this experiment can be found in the che226 lab handout, experiment #9, flame photometric analysis of. the flame photometer a traditional and simple method for determining sodium and potassium in biological fluids involves the. in a typical flame photometric experiment, a solution containing the relevant substance to be analysed is aspirated into the burner.. Flame Photometry Lab Report.
From www.tes.com
Flame Tests and Photometry GCSE AQA Teaching Resources Flame Photometry Lab Report by employing the chemical methods in use in this laboratory as a basis of comparison, it has been possible to establish the. flame photometry, also known as flame emission spectroscopy is a branch of spectroscopy that is concerned with the emission of characteristic radiation in flames by individual elements and the correlation of the emission intensity with the. Flame Photometry Lab Report.
From www.chegg.com
Solved Introduction Flame photometry, Experiment 10 Flame Photometry Lab Report the principle of flame photometer is based on the measurement of the emitted light intensity when a metal is introduced into. To determine the content of sodium and potassium by flame photometry. in a typical flame photometric experiment, a solution containing the relevant substance to be analysed is aspirated into the burner. the procedure for this experiment. Flame Photometry Lab Report.
From www.studypool.com
SOLUTION Flame photometry Studypool Flame Photometry Lab Report flame photometers (fps) have revolutionised the routine determination of ppm concentrations of key elements like. flame photometry is a highly empirical, in comparision to an absolute, method of analysis such as gravimetry. you are introducing barium sample in the flame photometer. by employing the chemical methods in use in this laboratory as a basis of comparison,. Flame Photometry Lab Report.