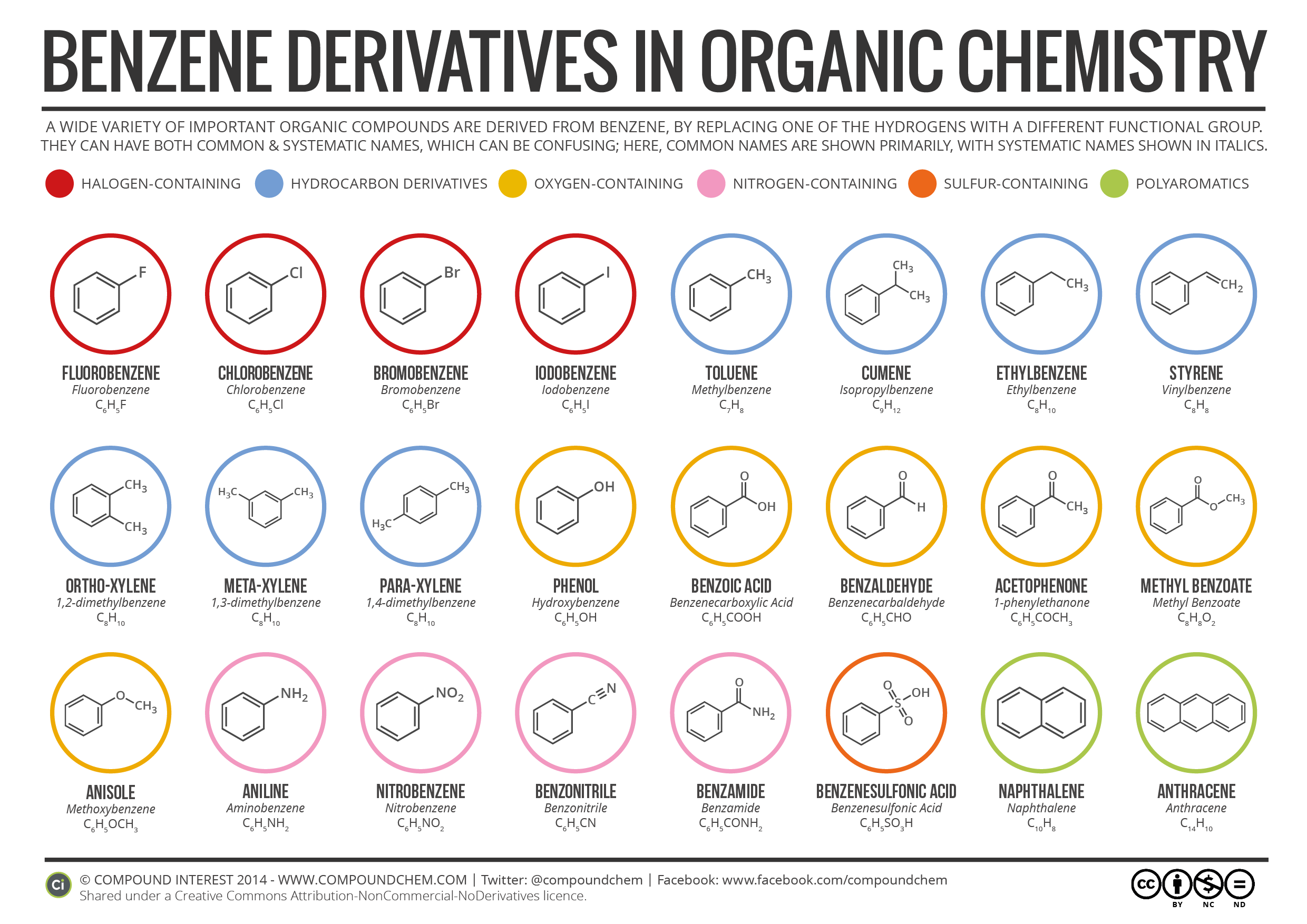
Benzene Ring In Organic Chemistry . Historically, because of the special aroma (sweet smelling) that. In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. Benzene has a molecular formula, c 6 h 6, whose structure is shown below. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Benzene (c₆h₆) is a fascinating molecule. As shown in illustration (1) below, benzene has six carbon atoms. Here's a deep dive into its structure and. We can verify the total number of pi electrons in. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. Benzene rings (c 6 h 6) are a special type of hydrocarbon. Each p orbital contains a single electron. In the case of the benzyl group, it.
from chemistry.com.pk
Here's a deep dive into its structure and. Each p orbital contains a single electron. As shown in illustration (1) below, benzene has six carbon atoms. Historically, because of the special aroma (sweet smelling) that. In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. We can verify the total number of pi electrons in. Benzene has a molecular formula, c 6 h 6, whose structure is shown below. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Benzene (c₆h₆) is a fascinating molecule. In the case of the benzyl group, it.
Benzene Derivatives and Their Nomenclature in Organic Chemistry
Benzene Ring In Organic Chemistry Benzene rings (c 6 h 6) are a special type of hydrocarbon. Benzene rings (c 6 h 6) are a special type of hydrocarbon. Each p orbital contains a single electron. Benzene (c₆h₆) is a fascinating molecule. Here's a deep dive into its structure and. In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. Historically, because of the special aroma (sweet smelling) that. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. As shown in illustration (1) below, benzene has six carbon atoms. We can verify the total number of pi electrons in. In the case of the benzyl group, it. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Benzene has a molecular formula, c 6 h 6, whose structure is shown below.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID223674 Benzene Ring In Organic Chemistry Benzene rings (c 6 h 6) are a special type of hydrocarbon. Benzene (c₆h₆) is a fascinating molecule. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. Benzene has a molecular formula, c 6 h 6, whose structure is shown below. We can verify the total number of pi electrons in. Each p orbital contains a. Benzene Ring In Organic Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Benzene ring Benzene Ring In Organic Chemistry Each p orbital contains a single electron. As shown in illustration (1) below, benzene has six carbon atoms. In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. Historically, because of the special aroma (sweet smelling) that. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called. Benzene Ring In Organic Chemistry.
From chemistrytalk.org
Benzene RingsStructure and Formula ChemTalk Benzene Ring In Organic Chemistry Benzene (c₆h₆) is a fascinating molecule. Benzene rings (c 6 h 6) are a special type of hydrocarbon. Each p orbital contains a single electron. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. In the case of the benzyl group, it. Here's a deep dive into its structure and. Benzene. Benzene Ring In Organic Chemistry.
From chemistry.com.pk
Benzene Derivatives and Their Nomenclature in Organic Chemistry Benzene Ring In Organic Chemistry Benzene rings (c 6 h 6) are a special type of hydrocarbon. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. In the case of the benzyl group, it. As shown in illustration (1) below, benzene has six carbon atoms. Here's a deep dive into its structure and. Bn), similar to. Benzene Ring In Organic Chemistry.
From socratic.org
How many sigma bonds are in benzene? Socratic Benzene Ring In Organic Chemistry Bn), similar to the phenyl group, is formed by manipulating the benzene ring. As shown in illustration (1) below, benzene has six carbon atoms. In the case of the benzyl group, it. Benzene rings (c 6 h 6) are a special type of hydrocarbon. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called. Benzene Ring In Organic Chemistry.
From www.chemistrystudent.com
Benzene Structure (ALevel) ChemistryStudent Benzene Ring In Organic Chemistry Here's a deep dive into its structure and. Benzene (c₆h₆) is a fascinating molecule. In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. Benzene rings (c 6 h 6) are a special type of hydrocarbon. Each p orbital contains a single electron. In the case of the benzyl group, it. Historically, because. Benzene Ring In Organic Chemistry.
From www.pngegg.com
Organic chemistry Molecule Organic compound Pyrimidine, benzene ring Benzene Ring In Organic Chemistry Benzene has a molecular formula, c 6 h 6, whose structure is shown below. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. As shown in illustration (1) below, benzene has six carbon atoms. Historically, because of the special aroma (sweet smelling) that. In the case of the benzyl group, it. Benzene rings (c 6 h. Benzene Ring In Organic Chemistry.
From www.chemistrylearner.com
Benzene Ring Formula and Structure Benzene Ring In Organic Chemistry Here's a deep dive into its structure and. We can verify the total number of pi electrons in. Benzene rings (c 6 h 6) are a special type of hydrocarbon. Each p orbital contains a single electron. Benzene has a molecular formula, c 6 h 6, whose structure is shown below. Historically, because of the special aroma (sweet smelling) that.. Benzene Ring In Organic Chemistry.
From chem.libretexts.org
Aromatic Chemistry LibreTexts Benzene Ring In Organic Chemistry Benzene rings (c 6 h 6) are a special type of hydrocarbon. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Each p orbital contains a single electron. In the case of the benzyl group, it. We can verify the total number of pi electrons in. Bn), similar to the phenyl. Benzene Ring In Organic Chemistry.
From www.anyrgb.com
Benzene Ring, borazine, delocalized Electron, aromatic Hydrocarbon Benzene Ring In Organic Chemistry Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Benzene (c₆h₆) is a fascinating molecule. Historically, because of the special aroma (sweet smelling) that. In the case of the benzyl group, it. Each p orbital contains a single electron. As shown in illustration (1) below, benzene has six carbon atoms. In. Benzene Ring In Organic Chemistry.
From techiescientist.com
Benzene Lewis Structure, Molecular Geometry, Hybridization, Polarity Benzene Ring In Organic Chemistry As shown in illustration (1) below, benzene has six carbon atoms. Here's a deep dive into its structure and. Each p orbital contains a single electron. Benzene rings (c 6 h 6) are a special type of hydrocarbon. In the case of the benzyl group, it. We can verify the total number of pi electrons in. Benzene has a molecular. Benzene Ring In Organic Chemistry.
From www.masterorganicchemistry.com
Rules for Aromaticity The 4 Key Factors Master Organic Chemistry Benzene Ring In Organic Chemistry In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. Benzene (c₆h₆) is a fascinating molecule. Benzene rings (c 6 h 6) are a special type of hydrocarbon. We can verify the total number of pi electrons in. Historically, because of the special aroma (sweet smelling) that. As shown in illustration (1) below,. Benzene Ring In Organic Chemistry.
From www.alamy.com
Benzene Ring structure .vector image Stock Vector Image & Art Alamy Benzene Ring In Organic Chemistry In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. We can verify the total number of pi electrons in. Each p orbital contains a single electron. In the case of the benzyl group, it. Benzene (c₆h₆) is a fascinating. Benzene Ring In Organic Chemistry.
From users.highland.edu
Organic Nomenclature II Benzene Ring In Organic Chemistry Here's a deep dive into its structure and. We can verify the total number of pi electrons in. Historically, because of the special aroma (sweet smelling) that. Benzene (c₆h₆) is a fascinating molecule. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Benzene rings (c 6 h 6) are a special. Benzene Ring In Organic Chemistry.
From www.pngwing.com
Organic chemistry Benzene Molecule, love chemistry, love, angle, ring Benzene Ring In Organic Chemistry In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. Benzene has a molecular formula, c 6 h 6, whose structure is shown below. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. We can verify the total number of pi electrons in. Each. Benzene Ring In Organic Chemistry.
From www.pngegg.com
Benzamide Organic chemistry Acid Derivative, benzene ring, angle, white Benzene Ring In Organic Chemistry Benzene (c₆h₆) is a fascinating molecule. Each p orbital contains a single electron. In the case of the benzyl group, it. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Benzene rings (c 6 h 6) are a special type of hydrocarbon. As shown in illustration (1) below, benzene has six. Benzene Ring In Organic Chemistry.
From www.youtube.com
benzene rings in organic chemistry YouTube Benzene Ring In Organic Chemistry Historically, because of the special aroma (sweet smelling) that. As shown in illustration (1) below, benzene has six carbon atoms. Each p orbital contains a single electron. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. Benzene (c₆h₆) is a fascinating molecule. In benzene, each p orbital is arranged at right angles (90°) to the plane. Benzene Ring In Organic Chemistry.
From ionscience.com
Benzene Ring Explained The Unique Structure of Benzene Benzene Ring In Organic Chemistry Benzene rings (c 6 h 6) are a special type of hydrocarbon. Here's a deep dive into its structure and. In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. We can verify the total number of pi electrons in. Bn), similar to the phenyl group, is formed by manipulating the benzene ring.. Benzene Ring In Organic Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Benzene Benzene Ring In Organic Chemistry In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. We can verify the total number of pi electrons in. Here's a deep dive into its structure and. As shown in illustration (1) below, benzene has six carbon atoms. In the case of the benzyl group, it. Historically, because of the special aroma. Benzene Ring In Organic Chemistry.
From openoregon.pressbooks.pub
4.4 Aromatic Hydrocarbons Introductory Organic Chemistry Benzene Ring In Organic Chemistry In the case of the benzyl group, it. Each p orbital contains a single electron. Benzene rings (c 6 h 6) are a special type of hydrocarbon. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. We can verify the total number of pi electrons in. As shown in illustration (1). Benzene Ring In Organic Chemistry.
From www.reddit.com
Aromatic hexazine rings — [N₆]⁴⁻ — an allnitrogen analogue of benzene Benzene Ring In Organic Chemistry Bn), similar to the phenyl group, is formed by manipulating the benzene ring. Benzene rings (c 6 h 6) are a special type of hydrocarbon. Historically, because of the special aroma (sweet smelling) that. We can verify the total number of pi electrons in. In benzene, each p orbital is arranged at right angles (90°) to the plane of the. Benzene Ring In Organic Chemistry.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Meta Benzene Ring In Organic Chemistry Each p orbital contains a single electron. Historically, because of the special aroma (sweet smelling) that. We can verify the total number of pi electrons in. As shown in illustration (1) below, benzene has six carbon atoms. Benzene (c₆h₆) is a fascinating molecule. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. In the case of. Benzene Ring In Organic Chemistry.
From www.alamy.com
Benzene is an organic chemical compound. Its molecule is composed of 6 Benzene Ring In Organic Chemistry Historically, because of the special aroma (sweet smelling) that. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. As shown in illustration (1) below, benzene has six carbon atoms. Benzene rings (c 6 h 6) are a special type of hydrocarbon. In the case of the benzyl group, it. Bn), similar. Benzene Ring In Organic Chemistry.
From www.masterorganicchemistry.com
Rules for Aromaticity The 4 Key Factors Master Organic Chemistry Benzene Ring In Organic Chemistry Historically, because of the special aroma (sweet smelling) that. Benzene (c₆h₆) is a fascinating molecule. Benzene has a molecular formula, c 6 h 6, whose structure is shown below. As shown in illustration (1) below, benzene has six carbon atoms. In the case of the benzyl group, it. Benzene rings (c 6 h 6) are a special type of hydrocarbon.. Benzene Ring In Organic Chemistry.
From www.masterorganicchemistry.com
Rules for Aromaticity The 4 Key Factors Master Organic Chemistry Benzene Ring In Organic Chemistry Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Each p orbital contains a single electron. Benzene rings (c 6 h 6) are a special type of hydrocarbon. Historically, because of the special aroma (sweet smelling) that. In the case of the benzyl group, it. Here's a deep dive into its. Benzene Ring In Organic Chemistry.
From byjus.com
Aromatic Compounds Definition, Example, Properties & Nomenclature Benzene Ring In Organic Chemistry In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. We can verify the total number of pi electrons in. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. Each p orbital contains a single electron. Benzene has a molecular formula, c 6 h 6, whose structure is shown. Benzene Ring In Organic Chemistry.
From www.youtube.com
How to Draw a Perfect Benzene Ring Structure YouTube Benzene Ring In Organic Chemistry Each p orbital contains a single electron. As shown in illustration (1) below, benzene has six carbon atoms. In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. Historically, because of the special aroma (sweet smelling) that. We can verify the total number of pi electrons in. Bn), similar to the phenyl group,. Benzene Ring In Organic Chemistry.
From chemistry.com.pk
Benzene Derivatives and Their Nomenclature in Organic Chemistry Benzene Ring In Organic Chemistry As shown in illustration (1) below, benzene has six carbon atoms. Benzene has a molecular formula, c 6 h 6, whose structure is shown below. In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. Each p orbital contains a single electron. Here's a deep dive into its structure and. We can verify. Benzene Ring In Organic Chemistry.
From www.alamy.com
Benzene is an organic chemical compound. Its molecule is composed of 6 Benzene Ring In Organic Chemistry Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Benzene rings (c 6 h 6) are a special type of hydrocarbon. We can verify the total number of pi electrons in. Benzene (c₆h₆) is a fascinating molecule. As shown in illustration (1) below, benzene has six carbon atoms. Here's a deep. Benzene Ring In Organic Chemistry.
From www.masterorganicchemistry.com
Birch Reduction of Aromatic Rings Master Organic Chemistry Benzene Ring In Organic Chemistry As shown in illustration (1) below, benzene has six carbon atoms. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. Benzene rings (c 6 h 6) are a special type of hydrocarbon. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Historically, because of the special aroma. Benzene Ring In Organic Chemistry.
From openpress.usask.ca
9.5. Nomenclature Introduction to Organic Chemistry Benzene Ring In Organic Chemistry Each p orbital contains a single electron. In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. Benzene (c₆h₆) is a fascinating molecule. In the case of the benzyl group, it. Historically, because of the special aroma (sweet smelling) that. We can verify the total number of pi electrons in. Benzene, c 6. Benzene Ring In Organic Chemistry.
From www.masterorganicchemistry.com
Introduction to Cycloalkanes Two Key Consequences of Ring Formation Benzene Ring In Organic Chemistry Benzene (c₆h₆) is a fascinating molecule. As shown in illustration (1) below, benzene has six carbon atoms. Benzene rings (c 6 h 6) are a special type of hydrocarbon. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Benzene has a molecular formula, c 6 h 6, whose structure is shown. Benzene Ring In Organic Chemistry.
From mavink.com
Structure Of Benzene Ring Benzene Ring In Organic Chemistry Benzene rings (c 6 h 6) are a special type of hydrocarbon. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. In the case of the benzyl group, it. Benzene (c₆h₆) is a fascinating molecule. Each p orbital contains a single electron. In benzene, each p orbital is arranged at right angles (90°) to the plane. Benzene Ring In Organic Chemistry.
From classnotes.org.in
Nomenclature of Simple Aromatic Compounds Chemistry, Class 11 Benzene Ring In Organic Chemistry In the case of the benzyl group, it. Historically, because of the special aroma (sweet smelling) that. In benzene, each p orbital is arranged at right angles (90°) to the plane of the ring. As shown in illustration (1) below, benzene has six carbon atoms. Bn), similar to the phenyl group, is formed by manipulating the benzene ring. Benzene has. Benzene Ring In Organic Chemistry.
From en.wikipedia.org
FileBenzene resonance structures.png Wikipedia Benzene Ring In Organic Chemistry We can verify the total number of pi electrons in. Benzene (c₆h₆) is a fascinating molecule. Benzene, c 6 h 6, is the simplest member of a large family of hydrocarbons, called aromatic hydrocarbons. Here's a deep dive into its structure and. Historically, because of the special aroma (sweet smelling) that. Benzene has a molecular formula, c 6 h 6,. Benzene Ring In Organic Chemistry.