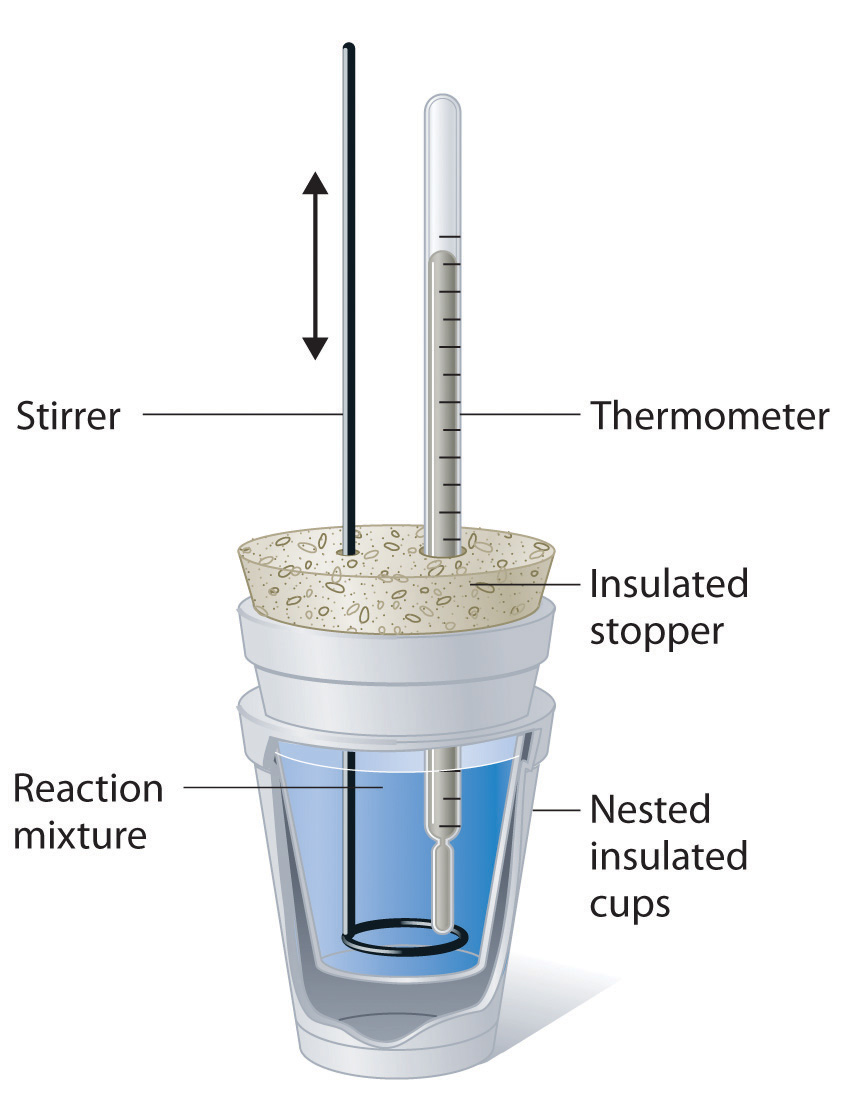
Calorimetry Lab Conclusion . Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. Lab session 9, experiment 8: Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s law of heat summation. In conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for different chemical reactions and. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. A vessel called a calorimeter is needed to hold the sub‐ stances under study.
from users.highland.edu
Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. Lab session 9, experiment 8: Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. In conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for different chemical reactions and. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s law of heat summation. A vessel called a calorimeter is needed to hold the sub‐ stances under study.
Calorimetry
Calorimetry Lab Conclusion Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. Lab session 9, experiment 8: In conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for different chemical reactions and. A vessel called a calorimeter is needed to hold the sub‐ stances under study. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s law of heat summation. Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Conclusion Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. Lab session 9, experiment 8: In conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for different chemical reactions and. Since it is difficult to measure the enthalpy of combustion of a metal directly,. Calorimetry Lab Conclusion.
From pdfprof.com
experiment 25 calorimetry lab report Calorimetry Lab Conclusion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Lab session 9, experiment 8: Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. A vessel called a calorimeter is needed to hold the sub‐. Calorimetry Lab Conclusion.
From studylib.net
Lab 33 calorimetry lab Calorimetry Lab Conclusion Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s law of heat summation. A vessel called a calorimeter is needed to hold the sub‐ stances under study. In conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for different. Calorimetry Lab Conclusion.
From dokumen.tips
(PDF) Experiment 25 Calorimetry Aaron N. Calorimetry Lab Conclusion A vessel called a calorimeter is needed to hold the sub‐ stances under study. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. Calorimetry, heat. Calorimetry Lab Conclusion.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Lab Conclusion Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample. Calorimetry Lab Conclusion.
From www.studocu.com
Lab 1 calorimetry of reactions and heat transfer chem 1302 Studocu Calorimetry Lab Conclusion Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s law of heat summation. Lab session 9, experiment 8: Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. A vessel called a calorimeter. Calorimetry Lab Conclusion.
From www.studocu.com
Calorimetry Prelab assignments Calorimetry Objective The purpose Calorimetry Lab Conclusion Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s law of heat summation. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Abstract the main purpose of the calorimetry experiment is. Calorimetry Lab Conclusion.
From wingle.jp
😂 Bomb calorimeter experiment conclusion. Experiment 1 A3 Bomb Calorimetry Lab Conclusion In conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for different chemical reactions and. Lab session 9, experiment 8: Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter. Calorimetry Lab Conclusion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Conclusion Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Lab session 9, experiment 8: In conclusion, the 6.03 calorimetry lab report provides valuable insights. Calorimetry Lab Conclusion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Conclusion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. A vessel called a calorimeter is needed to hold the sub‐ stances under study. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetery is. Calorimetry Lab Conclusion.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Lab Conclusion A vessel called a calorimeter is needed to hold the sub‐ stances under study. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying. Calorimetry Lab Conclusion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Conclusion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s law of heat summation. Calorimetry is the study of heat transferred in a chemical reaction,. Calorimetry Lab Conclusion.
From www.studocu.com
Post Lab 13 Calorimetry Heat of Reaction CHEM131L 3 010 Calorimetry Calorimetry Lab Conclusion Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. A vessel called a calorimeter is needed to hold the sub‐ stances under study.. Calorimetry Lab Conclusion.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Lab Conclusion Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s law of heat summation. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetry, heat of reaction specific heat is an intensive property of. Calorimetry Lab Conclusion.
From www.studocu.com
Calorimetry Lab Experiment 6. (1 week) (LCA) Calorimetry Purpose To Calorimetry Lab Conclusion A vessel called a calorimeter is needed to hold the sub‐ stances under study. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat.. Calorimetry Lab Conclusion.
From www.studocu.com
Caloremetry final Calorimetry Lab Report Calorimetry Objectives Calorimetry Lab Conclusion Lab session 9, experiment 8: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. A vessel called a calorimeter is needed to hold the sub‐ stances under study. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the. Calorimetry Lab Conclusion.
From www.scribd.com
Calorimetry Experiment Lab Report PDF Sodium Hydroxide Calorimetry Lab Conclusion Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s law of heat summation. Lab session 9, experiment 8: Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. In conclusion, the 6.03 calorimetry. Calorimetry Lab Conclusion.
From www.studocu.com
P calorimetry 25 lab report StuDocu Calorimetry Lab Conclusion Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s law of heat summation. A vessel called a calorimeter is needed to hold. Calorimetry Lab Conclusion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Conclusion Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. Lab session 9, experiment 8: A vessel called a calorimeter is needed to hold the. Calorimetry Lab Conclusion.
From users.highland.edu
Calorimetry Calorimetry Lab Conclusion Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s law of heat summation. Lab session 9, experiment 8: Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. Calorimetry is the study of. Calorimetry Lab Conclusion.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Conclusion Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Lab session 9, experiment 8: Abstract the main purpose of the calorimetry. Calorimetry Lab Conclusion.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry Lab Conclusion Lab session 9, experiment 8: Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. Since it is difficult to measure the enthalpy of combustion of. Calorimetry Lab Conclusion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Conclusion Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. A vessel called a calorimeter is needed to hold the sub‐ stances under study. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Since it. Calorimetry Lab Conclusion.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Lab Conclusion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. A vessel called a calorimeter is needed to hold the sub‐ stances under study. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetery is. Calorimetry Lab Conclusion.
From www.studocu.com
Calorimetry Lab Conclusions Questions Conclusion In a separate Calorimetry Lab Conclusion Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. A vessel called a calorimeter is needed to hold the sub‐ stances under study. Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. In. Calorimetry Lab Conclusion.
From www.scribd.com
Calorimetry Lab SE PDF Calorie Heat Capacity Calorimetry Lab Conclusion In conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for different chemical reactions and. A vessel called a calorimeter is needed to hold the sub‐ stances under study. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calorimetery is an application. Calorimetry Lab Conclusion.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Lab Conclusion Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is. Calorimetry Lab Conclusion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Conclusion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. In conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. Calorimetry Lab Conclusion.
From www.thinkswap.com
Calorimetry Lab Report PHYS1006 Foundations of Physics Curtin Calorimetry Lab Conclusion Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to. Calorimetry Lab Conclusion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Conclusion In conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for different chemical reactions and. Lab session 9, experiment 8: Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. Since it is difficult to measure the enthalpy of combustion of. Calorimetry Lab Conclusion.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Lab Conclusion Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. In conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for. Calorimetry Lab Conclusion.
From www.studocu.com
Formal Lab Report Calorimetry Experiment 25 CALORIMETRY Abstract Calorimetry Lab Conclusion Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the. Calorimetry Lab Conclusion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Conclusion In conclusion, the 6.03 calorimetry lab report provides valuable insights into the heat of reaction for different chemical reactions and. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure. Calorimetry Lab Conclusion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Lab Conclusion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. A vessel called a calorimeter is needed to hold the sub‐ stances under study. Calorimetry, heat of reaction specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. In conclusion,. Calorimetry Lab Conclusion.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Lab Conclusion Ideally, the calorimeter is a perfect insula‐ tor, that is, it should prevent the loss of heat from the system to. A vessel called a calorimeter is needed to hold the sub‐ stances under study. Since it is difficult to measure the enthalpy of combustion of a metal directly, in this lab it will be determined indirectly by applying hess’s. Calorimetry Lab Conclusion.