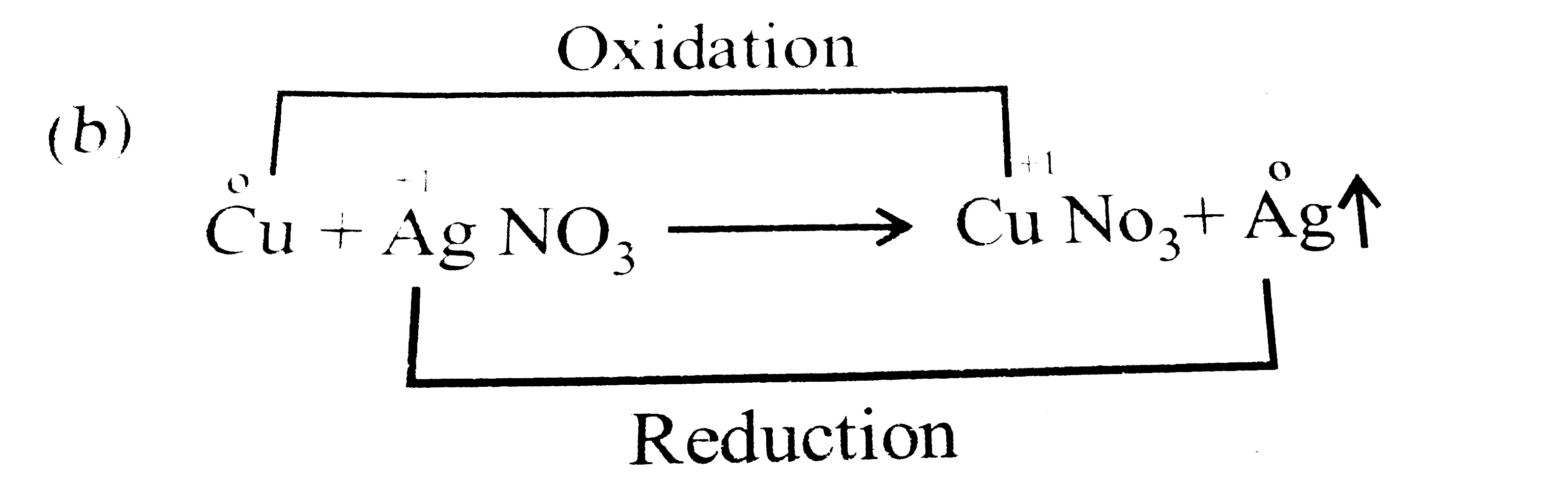
Copper Ii And Silver Nitrate Reaction . This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The solution gradually acquires the blue. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid.
from www.doubtnut.com
As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. The solution gradually acquires the blue. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate.
When a copper rod is dipped in aqueous silver nitrate solution , the c
Copper Ii And Silver Nitrate Reaction The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. The solution gradually acquires the blue. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and.
From www.chegg.com
Solved When silver nitrate reacts with copper, copper(II) Copper Ii And Silver Nitrate Reaction A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii). Copper Ii And Silver Nitrate Reaction.
From www.youtube.com
Balance AgNO3 + Cu = Cu(NO3)2 + Ag (Silver Nitrate and Copper) YouTube Copper Ii And Silver Nitrate Reaction Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. The solution gradually acquires the blue. One mole of solid copper plus two moles. Copper Ii And Silver Nitrate Reaction.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Ii And Silver Nitrate Reaction The solution gradually acquires the blue. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of. Copper Ii And Silver Nitrate Reaction.
From www.youtube.com
Displacement reactions of Silver nitrate by Copper YouTube Copper Ii And Silver Nitrate Reaction A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii). Copper Ii And Silver Nitrate Reaction.
From www.bartleby.com
Answered The balanced chemical equation for the… bartleby Copper Ii And Silver Nitrate Reaction As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The solution gradually acquires the blue. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of. Copper Ii And Silver Nitrate Reaction.
From www.youtube.com
Redox Reaction Copper and Silver Nitrate YouTube Copper Ii And Silver Nitrate Reaction A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the. Copper Ii And Silver Nitrate Reaction.
From www.doubtnut.com
When a copper rod is dipped in aqueous silver nitrate solution , the c Copper Ii And Silver Nitrate Reaction As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. One mole of solid copper plus two moles of aqueous silver nitrate produce one. Copper Ii And Silver Nitrate Reaction.
From www.sciencephoto.com
Copper reacting with silver nitrate Stock Image C030/8167 Science Copper Ii And Silver Nitrate Reaction As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. The reaction of copper wire. Copper Ii And Silver Nitrate Reaction.
From www.slideserve.com
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379 Copper Ii And Silver Nitrate Reaction A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. As demonstration of spontaneous chemical change,. Copper Ii And Silver Nitrate Reaction.
From www.slideserve.com
PPT Reaction of silver nitrate with copper to make silver and copper Copper Ii And Silver Nitrate Reaction One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. As demonstration of spontaneous chemical change, figure 17.2 shows the result of. Copper Ii And Silver Nitrate Reaction.
From www.sciencephoto.com
Silver nitratecopper displacement reaction Stock Image C002/7894 Copper Ii And Silver Nitrate Reaction One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. The solution gradually acquires the blue. This video captures a demonstration of a reaction. Copper Ii And Silver Nitrate Reaction.
From shaunmwilliams.com
Lecture 5 Presentation Copper Ii And Silver Nitrate Reaction One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. The solution gradually acquires the blue. A simple redox reaction occurs when copper metal is. Copper Ii And Silver Nitrate Reaction.
From www.slideserve.com
PPT Reaction of silver nitrate with copper to make silver and copper Copper Ii And Silver Nitrate Reaction Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. The reaction of copper wire with silver nitrate in aqueous solution shows. Copper Ii And Silver Nitrate Reaction.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Ii And Silver Nitrate Reaction This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into. Copper Ii And Silver Nitrate Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction silver nitrate copper Fundamental Copper Ii And Silver Nitrate Reaction One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to. Copper Ii And Silver Nitrate Reaction.
From express.adobe.com
Copper and Silver Nitrate Copper Ii And Silver Nitrate Reaction Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole. Copper Ii And Silver Nitrate Reaction.
From studylib.net
CopperSilver Nitrate Reaction Copper Ii And Silver Nitrate Reaction The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. A simple redox reaction occurs when copper metal is immersed in a solution of silver. Copper Ii And Silver Nitrate Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction silver nitrate copper Fundamental Copper Ii And Silver Nitrate Reaction The solution gradually acquires the blue. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. Start by identifying the reactants and products in the chemical reaction, which are copper. Copper Ii And Silver Nitrate Reaction.
From www.youtube.com
Copper and Silver Nitrate solution time lapse YouTube Copper Ii And Silver Nitrate Reaction The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. The solution gradually acquires the blue. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with. Copper Ii And Silver Nitrate Reaction.
From www.sciencephoto.com
Copper Reacts with Silver Nitrate, 2 of 6 Stock Image C028/1080 Copper Ii And Silver Nitrate Reaction A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The solution gradually acquires the blue. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a. Copper Ii And Silver Nitrate Reaction.
From www.numerade.com
The reaction between solid copper and aqueous silver nitrate produces Copper Ii And Silver Nitrate Reaction A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. One. Copper Ii And Silver Nitrate Reaction.
From lunblogbrun.blogspot.com
Copper Metal and Aqueous Silver Nitrate LunBlogBrun Copper Ii And Silver Nitrate Reaction Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. This video captures a demonstration of a reaction of copper wire with a solution. Copper Ii And Silver Nitrate Reaction.
From www.slideserve.com
PPT Salts PowerPoint Presentation ID1487768 Copper Ii And Silver Nitrate Reaction This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. One mole of solid copper plus. Copper Ii And Silver Nitrate Reaction.
From www.alamy.com
Displacement reaction of copper and silver nitrate solution. When Copper Ii And Silver Nitrate Reaction As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. The solution gradually acquires the blue. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. A simple redox reaction occurs when copper metal. Copper Ii And Silver Nitrate Reaction.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction of copper (Cu Copper Ii And Silver Nitrate Reaction Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. The reaction of. Copper Ii And Silver Nitrate Reaction.
From www.sciencephoto.com
Copper Reacts with Silver Nitrate, 4 of 6 Stock Image C028/1082 Copper Ii And Silver Nitrate Reaction The solution gradually acquires the blue. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. Start by identifying the reactants and products in the chemical reaction,. Copper Ii And Silver Nitrate Reaction.
From www.copperwiresuppliers.net
Copper Wire Silver Nitrate Copper Wire SuppliersCopper Wire Suppliers Copper Ii And Silver Nitrate Reaction This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii) nitrate and. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on. Copper Ii And Silver Nitrate Reaction.
From www.youtube.com
Copper And Silver Nitrate Make Silver And Copper (II) Nitrate YouTube Copper Ii And Silver Nitrate Reaction This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into. Copper Ii And Silver Nitrate Reaction.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Ii And Silver Nitrate Reaction As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. The solution gradually acquires the blue. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate. Copper Ii And Silver Nitrate Reaction.
From www.numerade.com
SOLVED When solid copper reacts with aqueous silver nitrate, the Copper Ii And Silver Nitrate Reaction A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. The solution gradually acquires the. Copper Ii And Silver Nitrate Reaction.
From lillyfersmcknight.blogspot.com
Copper Silver Nitrate Yields Copper Ii Nitrate Silver Copper Ii And Silver Nitrate Reaction The solution gradually acquires the blue. A simple redox reaction occurs when copper metal is immersed in a solution of silver nitrate. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper. Copper Ii And Silver Nitrate Reaction.
From www.youtube.com
Demonstration Displacement Reaction of Silver Nitrate and Copper Metal Copper Ii And Silver Nitrate Reaction The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate.. Copper Ii And Silver Nitrate Reaction.
From fphoto.photoshelter.com
displacement copper silver nitrate chemistry Fundamental Photographs Copper Ii And Silver Nitrate Reaction This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. One mole of solid copper plus two moles of aqueous silver nitrate produce one mole of copper (ii) nitrate plus two moles of solid. The solution gradually acquires the blue. A simple redox reaction occurs when copper metal is immersed in a solution. Copper Ii And Silver Nitrate Reaction.
From brainly.in
When copper turnings are added to Silver Nitrate solution a blue Copper Ii And Silver Nitrate Reaction The reaction of copper wire with silver nitrate in aqueous solution shows chemistry in action—delicate silver crystals grow on the wire. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. Start by identifying the reactants and products in the chemical reaction, which are copper reacting with silver nitrate to form copper (ii). Copper Ii And Silver Nitrate Reaction.
From www.slideserve.com
PPT Preparation of silver nitrate and its uses PowerPoint Copper Ii And Silver Nitrate Reaction As demonstration of spontaneous chemical change, figure 17.2 shows the result of immersing a coiled wire of copper into an aqueous solution. This video captures a demonstration of a reaction of copper wire with a solution of silver nitrate. The solution gradually acquires the blue. A simple redox reaction occurs when copper metal is immersed in a solution of silver. Copper Ii And Silver Nitrate Reaction.