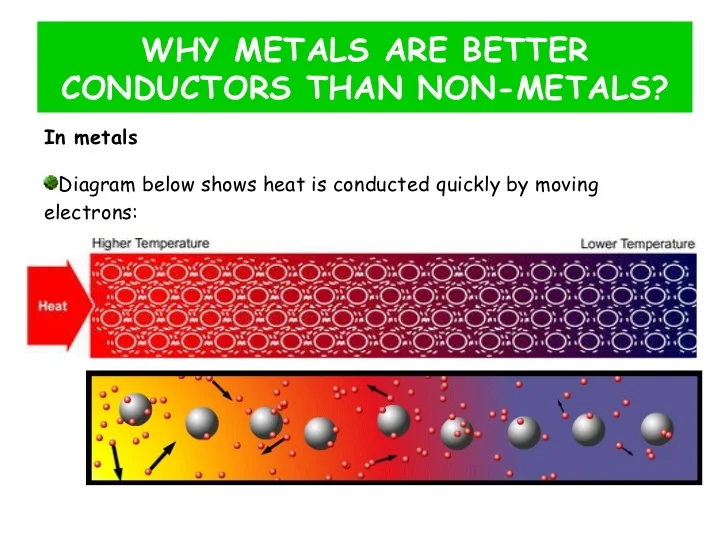
Why Does Copper Heat Up Fast . In metals, some of the electrons (often one per atom) are not. Pure copper has the best conductivity of any other metal. For example a 2kg copper wire with 150w dissipated as heat electrical power on the wire, will rise above ambient temperature δτ=50°c in 256 s. The consumed power in your. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. First, let me explain why metals generally conduct heat better than other solids do. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more.
from www.slideshare.net
In metals, some of the electrons (often one per atom) are not. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. For example a 2kg copper wire with 150w dissipated as heat electrical power on the wire, will rise above ambient temperature δτ=50°c in 256 s. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more. First, let me explain why metals generally conduct heat better than other solids do. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. The consumed power in your. Pure copper has the best conductivity of any other metal. For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water.
Chapter 24 Conduction
Why Does Copper Heat Up Fast In metals, some of the electrons (often one per atom) are not. In metals, some of the electrons (often one per atom) are not. For example a 2kg copper wire with 150w dissipated as heat electrical power on the wire, will rise above ambient temperature δτ=50°c in 256 s. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. The consumed power in your. First, let me explain why metals generally conduct heat better than other solids do. Pure copper has the best conductivity of any other metal.
From www.scribd.com
Lab 16 (Specific Heat of Copper) Heat Capacity Copper Why Does Copper Heat Up Fast For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. First, let me explain why metals generally conduct heat better than other solids do. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. Copper's thermal conductivity, or capacity. Why Does Copper Heat Up Fast.
From www.youtube.com
Heating a mixture of carbon and copper (II) oxide YouTube Why Does Copper Heat Up Fast Pure copper has the best conductivity of any other metal. For example a 2kg copper wire with 150w dissipated as heat electrical power on the wire, will rise above ambient temperature δτ=50°c in 256 s. In metals, some of the electrons (often one per atom) are not. For that reason, it is widely used for the manufacturing of heat exchangers,. Why Does Copper Heat Up Fast.
From mavink.com
Specific Heat Capacity Of Copper Why Does Copper Heat Up Fast Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. First, let me explain why metals generally conduct heat better than other solids do. The consumed power in your. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can. Why Does Copper Heat Up Fast.
From physicsexperiments.eu
Heat Dissipation Through a Copper Plate or How to Speed up Cooling of Why Does Copper Heat Up Fast The consumed power in your. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. First, let me explain why. Why Does Copper Heat Up Fast.
From www.researchgate.net
Structure of the copper surface after 47 h oxidation in air at 60 • C Why Does Copper Heat Up Fast Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. First, let me explain why metals generally conduct heat better than other solids do. For example a 2kg copper wire with 150w dissipated as heat electrical power on the wire, will rise above ambient temperature δτ=50°c in 256 s.. Why Does Copper Heat Up Fast.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Why Does Copper Heat Up Fast The consumed power in your. Pure copper has the best conductivity of any other metal. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. Making the base plate out of copper offers a few advantages over aluminum with the main. Why Does Copper Heat Up Fast.
From radianheatsinks.com
How do Copper Heat Pipes Work? Radian Why Does Copper Heat Up Fast Pure copper has the best conductivity of any other metal. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers.. Why Does Copper Heat Up Fast.
From www.youtube.com
Comparing Thermal Conductivity of Copper, Aluminium and Brass (Ice Why Does Copper Heat Up Fast For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. In metals, some of the electrons (often one per atom) are not. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to. Why Does Copper Heat Up Fast.
From www.researchgate.net
Temperaturedependent heat capacity of copper. Download Scientific Why Does Copper Heat Up Fast Pure copper has the best conductivity of any other metal. For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. First, let me explain why metals generally conduct heat better than other solids do. The consumed power in your. In metals, some of the electrons (often one per atom) are. Why Does Copper Heat Up Fast.
From www.researchgate.net
Copper heat intercepts, placed on the pulsetube cooler between the Why Does Copper Heat Up Fast First, let me explain why metals generally conduct heat better than other solids do. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. In metals, some of the electrons (often one per atom) are not. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater. Why Does Copper Heat Up Fast.
From exojchryu.blob.core.windows.net
Why Does Copper Sulphate Solution Turns Green at Alice McDonald blog Why Does Copper Heat Up Fast Pure copper has the best conductivity of any other metal. For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. In. Why Does Copper Heat Up Fast.
From www.heatell.com
Reliable Copper Heat Sink Manufacturer and Supplier Heatell Why Does Copper Heat Up Fast Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more. In metals, some of the electrons (often one per atom) are not. First, let me explain why metals generally conduct heat better than other solids do. Copper conducts heat very well and has a relatively low specific. Why Does Copper Heat Up Fast.
From www.researchgate.net
Schematic diagram of proposed copper heat spreader Download Why Does Copper Heat Up Fast For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. For example a 2kg copper wire with 150w dissipated as heat. Why Does Copper Heat Up Fast.
From www.alamy.com
copper, heating tube, sundries, coppers, heating tubes Stock Photo Alamy Why Does Copper Heat Up Fast Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. For example a 2kg copper wire with 150w dissipated as heat electrical power on the wire,. Why Does Copper Heat Up Fast.
From www.qats.com
Heat Spreading with Copper, Silicon and Heat Pipes Advanced Thermal Why Does Copper Heat Up Fast Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. Pure copper has the best conductivity of any other metal. For example a 2kg copper wire with 150w dissipated as heat electrical power on the wire, will rise above ambient temperature δτ=50°c in 256 s. In metals, some of. Why Does Copper Heat Up Fast.
From www.zetwerk.com
Copper Heat Sinks Uses Efficient Cooling for Electronics Why Does Copper Heat Up Fast In metals, some of the electrons (often one per atom) are not. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that. Why Does Copper Heat Up Fast.
From www.slideshare.net
Chapter 4 Why Does Copper Heat Up Fast Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more. For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. In metals, some of the electrons (often one per atom) are not. The consumed power in your.. Why Does Copper Heat Up Fast.
From mammothmemory.net
When metals are heated it reacts with oxygen to create flame Why Does Copper Heat Up Fast Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. Pure copper has the best conductivity of any other metal.. Why Does Copper Heat Up Fast.
From www.sciencephoto.com
Heating Copper Stock Image C002/8028 Science Photo Library Why Does Copper Heat Up Fast For example a 2kg copper wire with 150w dissipated as heat electrical power on the wire, will rise above ambient temperature δτ=50°c in 256 s. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than. Why Does Copper Heat Up Fast.
From www.reddit.com
Diamondback Nozzle + Copper Heat Block Volumetric Flow r/prusa3d Why Does Copper Heat Up Fast Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more. The consumed power in your. For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. For example a 2kg copper wire with 150w dissipated as heat electrical. Why Does Copper Heat Up Fast.
From www.youtube.com
Heat required to raise temperature of copper YouTube Why Does Copper Heat Up Fast First, let me explain why metals generally conduct heat better than other solids do. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators,. Why Does Copper Heat Up Fast.
From www.alamy.com
Copper Heating Pipe Joins Stock Photo Alamy Why Does Copper Heat Up Fast Pure copper has the best conductivity of any other metal. For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. In metals, some of the electrons (often one per atom) are not. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so. Why Does Copper Heat Up Fast.
From blog.thepipingmart.com
What Happens When Copper Is Heated In Air? Why Does Copper Heat Up Fast First, let me explain why metals generally conduct heat better than other solids do. The consumed power in your. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more. For example a 2kg copper wire with 150w dissipated as heat electrical power on the wire, will rise. Why Does Copper Heat Up Fast.
From www.thoughtco.com
Electrical Conductivity of Metals Why Does Copper Heat Up Fast Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. Pure copper has the best conductivity of any other metal.. Why Does Copper Heat Up Fast.
From instrumentationtools.com
Methods of Producing Voltage (Electricity) Inst Tools Why Does Copper Heat Up Fast Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more. First, let me explain why metals generally conduct heat better than other solids do. Pure copper. Why Does Copper Heat Up Fast.
From www.sciencephoto.com
Heating copper sulphate solution Stock Image A500/0588 Science Why Does Copper Heat Up Fast First, let me explain why metals generally conduct heat better than other solids do. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more. In metals, some of the electrons (often one per atom) are not. For that reason, it is widely used for the manufacturing of. Why Does Copper Heat Up Fast.
From byjus.com
Detailed description of reaction— Heating of copper powder in presence Why Does Copper Heat Up Fast The consumed power in your. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. First, let me explain why. Why Does Copper Heat Up Fast.
From www.engineeringtoolbox.com
Copper Tubes Heat Carrying Capacities Why Does Copper Heat Up Fast In metals, some of the electrons (often one per atom) are not. For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. The consumed power in your. First, let me explain why metals generally conduct heat better than other solids do. Making the base plate out of copper offers a. Why Does Copper Heat Up Fast.
From www.slideserve.com
PPT AQA GCSE 1a1 Heat Transfer PowerPoint Presentation ID281065 Why Does Copper Heat Up Fast First, let me explain why metals generally conduct heat better than other solids do. In metals, some of the electrons (often one per atom) are not. Making the base plate out of copper offers a few advantages over aluminum with the main reason being that copper transfers. Pure copper has the best conductivity of any other metal. Copper conducts heat. Why Does Copper Heat Up Fast.
From www.youtube.com
How To Solder Copper Pipe Guide) Plumbing 101 YouTube Why Does Copper Heat Up Fast For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. The consumed power in your. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. First, let me explain why metals. Why Does Copper Heat Up Fast.
From www.youtube.com
Heat transmission in a heat pipe and a copper pipe YouTube Why Does Copper Heat Up Fast In metals, some of the electrons (often one per atom) are not. First, let me explain why metals generally conduct heat better than other solids do. Pure copper has the best conductivity of any other metal. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more. For. Why Does Copper Heat Up Fast.
From www.yaclass.in
Effect of heat on solid, liquid and gases — lesson. Science State Board Why Does Copper Heat Up Fast For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. In metals, some of the electrons (often one per atom) are. Why Does Copper Heat Up Fast.
From www.fphoto.com
Fundamental Photographs The Art of Science Why Does Copper Heat Up Fast First, let me explain why metals generally conduct heat better than other solids do. The consumed power in your. Pure copper has the best conductivity of any other metal. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than that of aluminum, so copper can remove much more. For example a 2kg copper wire with 150w. Why Does Copper Heat Up Fast.
From americalplumbing.com
Gas flame heating copper piping AmeriCal Repipe and Plumbing, Inc. Why Does Copper Heat Up Fast In metals, some of the electrons (often one per atom) are not. Copper conducts heat very well and has a relatively low specific heat capacity, so it heats up and cools down extremely quickly (that's why cooking pots tend to have. The consumed power in your. Copper's thermal conductivity, or capacity to conduct heat, is about 60 percent greater than. Why Does Copper Heat Up Fast.
From www.slideshare.net
Chapter 24 Conduction Why Does Copper Heat Up Fast For example a 2kg copper wire with 150w dissipated as heat electrical power on the wire, will rise above ambient temperature δτ=50°c in 256 s. For that reason, it is widely used for the manufacturing of heat exchangers, air conditioning and refrigerators, and hot water. First, let me explain why metals generally conduct heat better than other solids do. Copper. Why Does Copper Heat Up Fast.