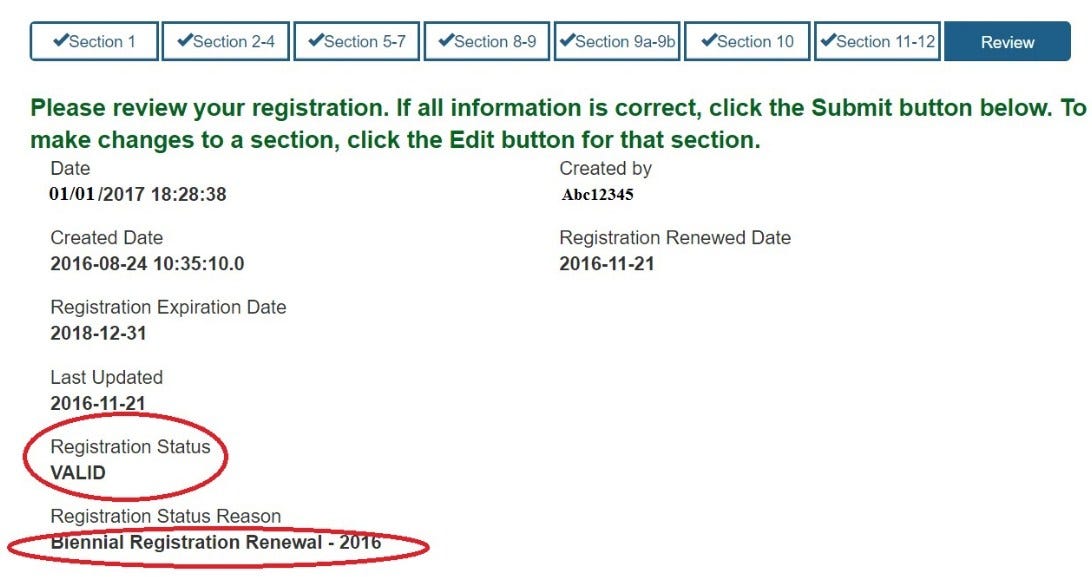
Fda Drug Listing And Registration . registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. Who must register and list. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). Included in this module are lessons on: welcome to fda direct. human drug establishment registration and drug listing compliance. you can enter a premarket submission number, a company name, registration or owner/operator number to. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. welcome to the module on who must register and list according to fda drls requirements.
from medium.com
human drug establishment registration and drug listing compliance. you can enter a premarket submission number, a company name, registration or owner/operator number to. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. Who must register and list. Included in this module are lessons on: welcome to fda direct. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). welcome to the module on who must register and list according to fda drls requirements. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those.
User Guide for FDA Food Facility Registration and FDA Registration
Fda Drug Listing And Registration welcome to fda direct. human drug establishment registration and drug listing compliance. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). welcome to fda direct. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. Included in this module are lessons on: Who must register and list. welcome to the module on who must register and list according to fda drls requirements. you can enter a premarket submission number, a company name, registration or owner/operator number to.
From medium.com
User Guide for FDA Food Facility Registration and FDA Registration Fda Drug Listing And Registration welcome to fda direct. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). you can enter a premarket submission number, a company name, registration or owner/operator number to. human drug establishment registration. Fda Drug Listing And Registration.
From www.meddevicecorp.com
US FDA Registration Service of Food, Drugs, Medical Devices Fda Drug Listing And Registration there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. welcome to the module on who must register and list according to fda drls requirements. Included in this module are lessons on: Who must register and list. you can enter a premarket submission number, a company name, registration. Fda Drug Listing And Registration.
From fdaspecialist.com
FDA Specialist News Fda Drug Listing And Registration registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). welcome to fda direct. human drug establishment registration and drug listing compliance. Who must register and list. there are three steps, or submissions,. Fda Drug Listing And Registration.
From www.signnow.com
Form FDA 1572 PDF Food and Drug Administration Fill Out and Sign Fda Drug Listing And Registration establishment registration and drug listing data are submitted electronically using structured product labeling (spl). registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. welcome to fda direct. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. Included. Fda Drug Listing And Registration.
From www.fdahelp.us
FDA Registration Dietary Supplements Fda Drug Listing And Registration you can enter a premarket submission number, a company name, registration or owner/operator number to. welcome to fda direct. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. human drug establishment registration and drug listing compliance. registration and listing provides fda with the location of. Fda Drug Listing And Registration.
From www.fdahelp.us
How to Get FDA Approval FDAHelp USA Fda Drug Listing And Registration human drug establishment registration and drug listing compliance. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. welcome to the module on who must register and list according to fda drls requirements. Included in this module are lessons on: welcome to fda direct. you can enter. Fda Drug Listing And Registration.
From www.pkguis.com
Quality Documents — PKG User Interface Solutions Fda Drug Listing And Registration you can enter a premarket submission number, a company name, registration or owner/operator number to. Who must register and list. human drug establishment registration and drug listing compliance. welcome to the module on who must register and list according to fda drls requirements. establishment registration and drug listing data are submitted electronically using structured product labeling. Fda Drug Listing And Registration.
From www.fdabasics.com
All You Need To Know Responsibilities Of An FDA US Agent FDABasics Fda Drug Listing And Registration establishment registration and drug listing data are submitted electronically using structured product labeling (spl). you can enter a premarket submission number, a company name, registration or owner/operator number to. human drug establishment registration and drug listing compliance. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda.. Fda Drug Listing And Registration.
From www.fdabasics.com
US FDA Registration is required for your product ? Understanding US FDA Fda Drug Listing And Registration registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. Who must register and list. welcome to fda direct. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). you can enter a premarket submission number, a company name, registration or owner/operator number to.. Fda Drug Listing And Registration.
From digitalhealthcentral.com
Understand the differences between FDA Approved vs Cleared and Fda Drug Listing And Registration welcome to the module on who must register and list according to fda drls requirements. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). Included in this module are lessons on: welcome to fda direct. registration and listing provides fda with the location of medical device establishments and the devices manufactured. Fda Drug Listing And Registration.
From vetmed.ucsd.edu
Federal Regulatory Agencies Fda Drug Listing And Registration there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. welcome to the module on who must register and list according to fda drls requirements. Included in this module are lessons on: Who must register and list. registration and listing provides fda with the location of medical device. Fda Drug Listing And Registration.
From www.libertymanagement.us
FDA Registration Number FDA Registration Search Fda Drug Listing And Registration you can enter a premarket submission number, a company name, registration or owner/operator number to. Who must register and list. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). Included in this module are. Fda Drug Listing And Registration.
From mungfali.com
FDA Drug Approval Process Chart Fda Drug Listing And Registration you can enter a premarket submission number, a company name, registration or owner/operator number to. welcome to fda direct. human drug establishment registration and drug listing compliance. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). there are three steps, or submissions, that are needed to register an establishment and. Fda Drug Listing And Registration.
From www.indiamart.com
US FDA Registration Services at Rs 5000/unit Main Kakrola Road New Fda Drug Listing And Registration welcome to fda direct. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). human drug establishment registration and drug listing compliance. welcome to the module on who must register and list according to fda drls requirements. registration and listing provides fda with the location of medical device establishments and the. Fda Drug Listing And Registration.
From www.researchgate.net
Peptidebased drugs approved by the Food Drug Administration (FDA Fda Drug Listing And Registration you can enter a premarket submission number, a company name, registration or owner/operator number to. human drug establishment registration and drug listing compliance. welcome to fda direct. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. Included in this module are lessons on: Who must register. Fda Drug Listing And Registration.
From www.fdahelp.us
How to get FDA Registration FDA Certificate Fda Drug Listing And Registration Who must register and list. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. . Fda Drug Listing And Registration.
From www.fda.gov
Electronic Drug Registration and Listing Using CDER Direct 10/22/2019 Fda Drug Listing And Registration Who must register and list. Included in this module are lessons on: welcome to fda direct. you can enter a premarket submission number, a company name, registration or owner/operator number to. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). human drug establishment registration and drug listing compliance. welcome to. Fda Drug Listing And Registration.
From exojyybsv.blob.core.windows.net
Fda Drug Facility Listing at Debra Milligan blog Fda Drug Listing And Registration there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. you can enter a premarket submission number, a company name, registration or owner/operator number to. human drug establishment registration and drug listing compliance. establishment registration and drug listing data are submitted electronically using structured product labeling (spl).. Fda Drug Listing And Registration.
From www.slideserve.com
PPT National Drug Code (NDC) PowerPoint Presentation ID4414874 Fda Drug Listing And Registration you can enter a premarket submission number, a company name, registration or owner/operator number to. Included in this module are lessons on: welcome to fda direct. Who must register and list. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. welcome to the module on who. Fda Drug Listing And Registration.
From medicaldeviceacademy.com
Private Labeled Devices with FDA Approval Medical Device Academy Fda Drug Listing And Registration human drug establishment registration and drug listing compliance. welcome to the module on who must register and list according to fda drls requirements. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. establishment registration and drug listing data are submitted electronically using structured product labeling (spl).. Fda Drug Listing And Registration.
From www.fdahelp.us
FDA Registration Number and other FDA Requirements FDAHELP.US Fda Drug Listing And Registration registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. human drug establishment registration and drug listing compliance. welcome to the module on who must register and list according to fda drls requirements. Who must register and list. welcome to fda direct. Included in this module are lessons. Fda Drug Listing And Registration.
From biovet-alquermes.com
FDA Certificate Fda Drug Listing And Registration welcome to the module on who must register and list according to fda drls requirements. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). welcome to fda direct. human drug establishment registration and drug listing compliance. Included in this module are lessons on: there are three steps, or submissions, that. Fda Drug Listing And Registration.
From www.asphalion.com
FDA aims to start deactivating outdated drug listing records to ensure Fda Drug Listing And Registration registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. Who must register and list. you can enter a premarket submission number, a company name, registration or owner/operator number to. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda.. Fda Drug Listing And Registration.
From www.slideshare.net
FDA Drug Registration Renewal and FDA Certificates Number Service F… Fda Drug Listing And Registration welcome to fda direct. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. you can enter a premarket submission number, a company name, registration or owner/operator number to. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). Who must register and. Fda Drug Listing And Registration.
From www.fdabasics.com
An Overview of FDA Requirements for OTC Drugs (Over the Counter Fda Drug Listing And Registration welcome to fda direct. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. Included in this module are lessons on: there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. you can enter a premarket submission number, a. Fda Drug Listing And Registration.
From www.reedtech.com
inar Recording FDA Drug Listing Requirements for Manufacturers Fda Drug Listing And Registration there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. Who must register and list. welcome to fda direct. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. you can enter a premarket submission number, a company name,. Fda Drug Listing And Registration.
From heng-li-yuan.com
U.S Food and Drug Administration Food Facility Registration(FDA FFR Fda Drug Listing And Registration welcome to fda direct. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. Who must register and list. there are three steps, or submissions, that are needed to register an establishment and list. Fda Drug Listing And Registration.
From www.28ceramics.com
Best Fda Registration Certificate Manufacture Fda Drug Listing And Registration welcome to the module on who must register and list according to fda drls requirements. there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. welcome to fda direct. you can enter a premarket submission number, a company name, registration or owner/operator number to. Who must register. Fda Drug Listing And Registration.
From ecommed.vn
The US Food and Drug Administration FDA Certificate Of Registration Fda Drug Listing And Registration Who must register and list. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. Included in this module are lessons on: there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. you can enter a premarket submission number, a. Fda Drug Listing And Registration.
From nogatupharmaceuticals.com
About Us Nogatu Pharmaceuticals Inc. Fda Drug Listing And Registration registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. human drug establishment registration and drug listing compliance. you can enter a premarket submission number, a company name, registration or owner/operator number to. Included in this module are lessons on: Who must register and list. welcome to the. Fda Drug Listing And Registration.
From www.pharmacompass.com
FDA & EMA’s New Drug Approvals (Mid2021 Recap) Radio Data Compilation Fda Drug Listing And Registration establishment registration and drug listing data are submitted electronically using structured product labeling (spl). welcome to the module on who must register and list according to fda drls requirements. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. Who must register and list. you can enter a. Fda Drug Listing And Registration.
From www.researchgate.net
Current FDAapproved drugs and drugs in clinical trials Fda Drug Listing And Registration welcome to fda direct. you can enter a premarket submission number, a company name, registration or owner/operator number to. Who must register and list. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. there are three steps, or submissions, that are needed to register an establishment and. Fda Drug Listing And Registration.
From www.fdalisting.com
Sample FDA Registration Certificates Fda Drug Listing And Registration there are three steps, or submissions, that are needed to register an establishment and list a drug with fda. welcome to the module on who must register and list according to fda drls requirements. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). Included in this module are lessons on: human. Fda Drug Listing And Registration.
From www.researchgate.net
List of FDAapproved drugs (2020) along with their results for Fda Drug Listing And Registration you can enter a premarket submission number, a company name, registration or owner/operator number to. establishment registration and drug listing data are submitted electronically using structured product labeling (spl). human drug establishment registration and drug listing compliance. registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. . Fda Drug Listing And Registration.
From www.libertymanagement.us
FDA Registration Number FDA Registration Search Fda Drug Listing And Registration registration and listing provides fda with the location of medical device establishments and the devices manufactured at those. welcome to fda direct. Included in this module are lessons on: you can enter a premarket submission number, a company name, registration or owner/operator number to. establishment registration and drug listing data are submitted electronically using structured product. Fda Drug Listing And Registration.