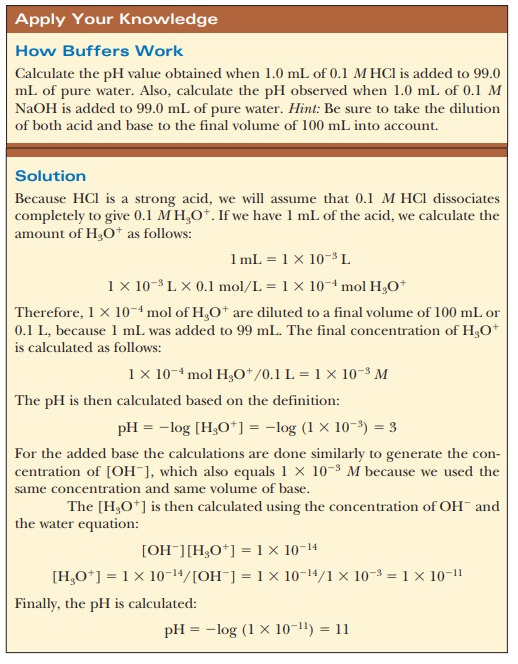
Buffer In Experiments . Clearly, the buffer minimizes the impact of the added protons compared to the pure water. Remember to return it to the storage solution as soon you. Buffer systems are also of particular importance to experimental cell biology. Choose a buffer that is compatible with your. Choose a buffer with a pka that matches the ph of your intended experiment. Choose a buffer that doesn’t react with your analytes. In this laboratory you will cover the basics of buffer preparation and test the buffering. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Biochemical experiments routinely require a buffer. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. This buffer helps maintain the blood ph around 7.4, with carbonic acid acting as the weak acid and bicarbonate as the weak base. Thus, the pure water solution sees its ph fall from 7 to 2 (5 ph units), whereas the buffered solution saw its ph drop from 4.76 to 4.74 (0.02 ph units). So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18.
from www.brainkart.com
Choose a buffer that doesn’t react with your analytes. Choose a buffer with a pka that matches the ph of your intended experiment. Clearly, the buffer minimizes the impact of the added protons compared to the pure water. This buffer helps maintain the blood ph around 7.4, with carbonic acid acting as the weak acid and bicarbonate as the weak base. Thus, the pure water solution sees its ph fall from 7 to 2 (5 ph units), whereas the buffered solution saw its ph drop from 4.76 to 4.74 (0.02 ph units). Buffer systems are also of particular importance to experimental cell biology. Biochemical experiments routinely require a buffer. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. In this laboratory you will cover the basics of buffer preparation and test the buffering. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74.
Buffers
Buffer In Experiments Biochemical experiments routinely require a buffer. Biochemical experiments routinely require a buffer. Choose a buffer that doesn’t react with your analytes. Remember to return it to the storage solution as soon you. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Thus, the pure water solution sees its ph fall from 7 to 2 (5 ph units), whereas the buffered solution saw its ph drop from 4.76 to 4.74 (0.02 ph units). So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. This buffer helps maintain the blood ph around 7.4, with carbonic acid acting as the weak acid and bicarbonate as the weak base. Choose a buffer that is compatible with your. Clearly, the buffer minimizes the impact of the added protons compared to the pure water. In this laboratory you will cover the basics of buffer preparation and test the buffering. Buffer systems are also of particular importance to experimental cell biology. Choose a buffer with a pka that matches the ph of your intended experiment. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids.
From studylib.net
Experiment 7 Preparation of a Buffer Buffer In Experiments Choose a buffer that doesn’t react with your analytes. Buffer systems are also of particular importance to experimental cell biology. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Clearly, the buffer minimizes the impact of the added protons compared to the pure water. Buffers and how they control hydrogen ion concentrations, a brief. Buffer In Experiments.
From www.youtube.com
How to Make and pH Buffers YouTube Buffer In Experiments Buffer systems are also of particular importance to experimental cell biology. In this laboratory you will cover the basics of buffer preparation and test the buffering. Remember to return it to the storage solution as soon you. Choose a buffer that is compatible with your. Choose a buffer with a pka that matches the ph of your intended experiment. Buffers. Buffer In Experiments.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer In Experiments Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Choose a buffer that is compatible with your. Thus, the pure water solution sees its ph fall from 7 to 2 (5 ph units), whereas the buffered solution saw its ph drop from 4.76 to 4.74 (0.02 ph. Buffer In Experiments.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Buffer In Experiments Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Choose a buffer with a pka that matches the ph of your intended experiment. Buffer systems are also of particular importance to experimental cell biology. This buffer helps maintain the blood ph around 7.4, with carbonic acid acting. Buffer In Experiments.
From www.youtube.com
Lab 8 Acids, Bases, and Buffers experiment YouTube Buffer In Experiments This buffer helps maintain the blood ph around 7.4, with carbonic acid acting as the weak acid and bicarbonate as the weak base. Clearly, the buffer minimizes the impact of the added protons compared to the pure water. Biochemical experiments routinely require a buffer. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Buffers. Buffer In Experiments.
From www.pinterest.com
Buffer Buffer solution, Easy science, Flashcards Buffer In Experiments Clearly, the buffer minimizes the impact of the added protons compared to the pure water. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Choose a buffer that doesn’t react with your. Buffer In Experiments.
From magmastory.blogspot.com
Which Of The Following Represents A Buffer System? magmastory Buffer In Experiments So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. Clearly, the buffer minimizes the impact of the added protons compared to the pure water. Choose a buffer that doesn’t react with your analytes. Buffer systems are also of particular importance to experimental cell biology. Choose a buffer that is compatible with your.. Buffer In Experiments.
From www.flinnsci.ca
Buffers in Household Products—Advanced Inquiry Laboratory Kit Flinn Buffer In Experiments This buffer helps maintain the blood ph around 7.4, with carbonic acid acting as the weak acid and bicarbonate as the weak base. Buffer systems are also of particular importance to experimental cell biology. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Remember to return it to the storage solution as soon you.. Buffer In Experiments.
From www.chegg.com
Solved Preparation of Buffer Solution Experiment I uploaded Buffer In Experiments Choose a buffer that is compatible with your. Biochemical experiments routinely require a buffer. Choose a buffer that doesn’t react with your analytes. Clearly, the buffer minimizes the impact of the added protons compared to the pure water. In this laboratory you will cover the basics of buffer preparation and test the buffering. Buffers and how they control hydrogen ion. Buffer In Experiments.
From www.youtube.com
14.10 Buffers Solutions that Resist pH Change YouTube Buffer In Experiments Thus, the pure water solution sees its ph fall from 7 to 2 (5 ph units), whereas the buffered solution saw its ph drop from 4.76 to 4.74 (0.02 ph units). In this laboratory you will cover the basics of buffer preparation and test the buffering. So, a hydrofluoric acid buffer would work best in a buffer range of around. Buffer In Experiments.
From www.thoughtco.com
What Is a Buffer and How Does It Work? Buffer In Experiments Remember to return it to the storage solution as soon you. Choose a buffer that is compatible with your. Biochemical experiments routinely require a buffer. Buffer systems are also of particular importance to experimental cell biology. So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. Buffers and how they control hydrogen ion. Buffer In Experiments.
From pdfprof.com
buffer capacity experiment procedure Buffer In Experiments Clearly, the buffer minimizes the impact of the added protons compared to the pure water. Choose a buffer that is compatible with your. Choose a buffer with a pka that matches the ph of your intended experiment. Choose a buffer that doesn’t react with your analytes. This buffer helps maintain the blood ph around 7.4, with carbonic acid acting as. Buffer In Experiments.
From app.jove.com
Buffers, Buffer Components and Buffer Action Chemistry JoVe Buffer In Experiments Remember to return it to the storage solution as soon you. Buffer systems are also of particular importance to experimental cell biology. So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. Choose a buffer that is compatible with your. Choose a buffer with a pka that matches the ph of your intended. Buffer In Experiments.
From www.pdfprof.com
types of buffer solution in chemistry Buffer In Experiments Choose a buffer that is compatible with your. Choose a buffer that doesn’t react with your analytes. Choose a buffer with a pka that matches the ph of your intended experiment. Remember to return it to the storage solution as soon you. So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. Buffer. Buffer In Experiments.
From webmis.highland.cc.il.us
Buffers Buffer In Experiments This buffer helps maintain the blood ph around 7.4, with carbonic acid acting as the weak acid and bicarbonate as the weak base. So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Remember to return it to. Buffer In Experiments.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and Buffer In Experiments Choose a buffer with a pka that matches the ph of your intended experiment. In this laboratory you will cover the basics of buffer preparation and test the buffering. Remember to return it to the storage solution as soon you. Buffer systems are also of particular importance to experimental cell biology. Clearly, the buffer minimizes the impact of the added. Buffer In Experiments.
From dandkmotorsports.com
Hepes Buffer Recipe Ph 7 2 Dandk Organizer Buffer In Experiments Choose a buffer that is compatible with your. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Buffer systems are also of particular importance to experimental cell biology. So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. In this laboratory you will cover the basics of. Buffer In Experiments.
From www.studocu.com
Buffers Worksheet Working with Buffers Experiment Determining the pH Buffer In Experiments Choose a buffer with a pka that matches the ph of your intended experiment. So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. Clearly, the buffer minimizes the impact of the added protons compared to the pure water. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role. Buffer In Experiments.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Buffer In Experiments By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Biochemical experiments routinely require a buffer. Choose a buffer that doesn’t react with your analytes. Choose a buffer with a pka that matches the ph of your intended experiment. This buffer helps maintain the blood ph around 7.4, with carbonic acid acting as the weak. Buffer In Experiments.
From studylib.net
EXPERIMENT 1 Buffers Buffer In Experiments Remember to return it to the storage solution as soon you. Choose a buffer that is compatible with your. Clearly, the buffer minimizes the impact of the added protons compared to the pure water. Buffer systems are also of particular importance to experimental cell biology. Thus, the pure water solution sees its ph fall from 7 to 2 (5 ph. Buffer In Experiments.
From www.studocu.com
Experiment 8 Buffers Introduction and Procedure Experiment 8 Buffers Buffer In Experiments By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Choose a buffer that is compatible with your. So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. Buffer systems are also of particular importance to experimental cell biology. Choose a buffer that doesn’t react with your analytes.. Buffer In Experiments.
From studylib.net
experiment 16 buffer solutions Buffer In Experiments Choose a buffer that doesn’t react with your analytes. Thus, the pure water solution sees its ph fall from 7 to 2 (5 ph units), whereas the buffered solution saw its ph drop from 4.76 to 4.74 (0.02 ph units). Clearly, the buffer minimizes the impact of the added protons compared to the pure water. Biochemical experiments routinely require a. Buffer In Experiments.
From www.pinterest.com
Pin on Download Buffer In Experiments Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Choose a buffer that is compatible with your. Biochemical experiments routinely require a buffer. So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. Clearly, the buffer minimizes the impact of. Buffer In Experiments.
From www.youtube.com
Buffers Animation YouTube Buffer In Experiments Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Thus, the pure water solution sees its ph fall from 7 to 2 (5 ph units), whereas the buffered solution saw its ph drop from 4.76 to 4.74 (0.02 ph units). This buffer helps maintain the blood ph. Buffer In Experiments.
From www.slideshare.net
Buffer system Buffer In Experiments Buffer systems are also of particular importance to experimental cell biology. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Choose a buffer with a pka that matches the ph of your intended experiment. This buffer helps maintain the blood ph around 7.4, with carbonic acid acting. Buffer In Experiments.
From www.pinterest.com.au
Buffer solutions Buffer solution, Chemistry lessons, Ap chemistry Buffer In Experiments Thus, the pure water solution sees its ph fall from 7 to 2 (5 ph units), whereas the buffered solution saw its ph drop from 4.76 to 4.74 (0.02 ph units). In this laboratory you will cover the basics of buffer preparation and test the buffering. Biochemical experiments routinely require a buffer. This buffer helps maintain the blood ph around. Buffer In Experiments.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Buffer In Experiments This buffer helps maintain the blood ph around 7.4, with carbonic acid acting as the weak acid and bicarbonate as the weak base. Biochemical experiments routinely require a buffer. Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. Choose a buffer that is compatible with your. Buffer. Buffer In Experiments.
From www.vernier.com
Buffers > Experiment 19 from Advanced Chemistry with Vernier Buffer In Experiments Choose a buffer with a pka that matches the ph of your intended experiment. Choose a buffer that doesn’t react with your analytes. Biochemical experiments routinely require a buffer. In this laboratory you will cover the basics of buffer preparation and test the buffering. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Buffer. Buffer In Experiments.
From www.brainkart.com
Buffers Buffer In Experiments Thus, the pure water solution sees its ph fall from 7 to 2 (5 ph units), whereas the buffered solution saw its ph drop from 4.76 to 4.74 (0.02 ph units). Choose a buffer with a pka that matches the ph of your intended experiment. In this laboratory you will cover the basics of buffer preparation and test the buffering.. Buffer In Experiments.
From studylib.net
Buffer Capacity Buffer In Experiments This buffer helps maintain the blood ph around 7.4, with carbonic acid acting as the weak acid and bicarbonate as the weak base. In this laboratory you will cover the basics of buffer preparation and test the buffering. Remember to return it to the storage solution as soon you. Thus, the pure water solution sees its ph fall from 7. Buffer In Experiments.
From www.researchgate.net
(PDF) A Discovery Chemistry Experiment on Buffers Buffer In Experiments Choose a buffer that is compatible with your. Biochemical experiments routinely require a buffer. So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. In this laboratory you will cover the basics of buffer preparation and test the. Buffer In Experiments.
From www.slideserve.com
PPT Experiment 7 Preparation and Properties of Buffers PowerPoint Buffer In Experiments Clearly, the buffer minimizes the impact of the added protons compared to the pure water. Choose a buffer with a pka that matches the ph of your intended experiment. Choose a buffer that doesn’t react with your analytes. Remember to return it to the storage solution as soon you. By contrast, the acetate buffer’s ph after adding the same amount. Buffer In Experiments.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent Buffer In Experiments So, a hydrofluoric acid buffer would work best in a buffer range of around ph = 3.18. Clearly, the buffer minimizes the impact of the added protons compared to the pure water. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Choose a buffer that is compatible with your. Biochemical experiments routinely require a. Buffer In Experiments.
From www.youtube.com
Chapter 16 Buffers Some Buffer Experiments with Household Items Buffer In Experiments Buffers and how they control hydrogen ion concentrations, a brief explanation of the role of water and equilibrium constants of weak acids. By contrast, the acetate buffer’s ph after adding the same amount of hcl is 4.74. Biochemical experiments routinely require a buffer. In this laboratory you will cover the basics of buffer preparation and test the buffering. So, a. Buffer In Experiments.
From studylib.net
Buffer Solutions Buffer In Experiments Remember to return it to the storage solution as soon you. Choose a buffer that is compatible with your. Choose a buffer with a pka that matches the ph of your intended experiment. Choose a buffer that doesn’t react with your analytes. This buffer helps maintain the blood ph around 7.4, with carbonic acid acting as the weak acid and. Buffer In Experiments.