Calorimetry Lab Activity C . The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. Measure the amount of heat involved in frostbite. Calorimetry is the science of measuring heat flow. The amount of heat that flows in or out of a. One way to do this is to place a sample of the. Measuring the latent heat of fusion of water, and measuring. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. As part of this lab, you will: The specific heat of a substance is easily determined by comparing it to that of water. Calorimeters can be used to find a substance’s specific heat. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. In this lab, you will do two classic calorimetry experiments:
from www.studocu.com
When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. The specific heat of a substance is easily determined by comparing it to that of water. The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. Calorimeters can be used to find a substance’s specific heat. Measure the amount of heat involved in frostbite. Calorimetry is the science of measuring heat flow. One way to do this is to place a sample of the. As part of this lab, you will: Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Measuring the latent heat of fusion of water, and measuring.
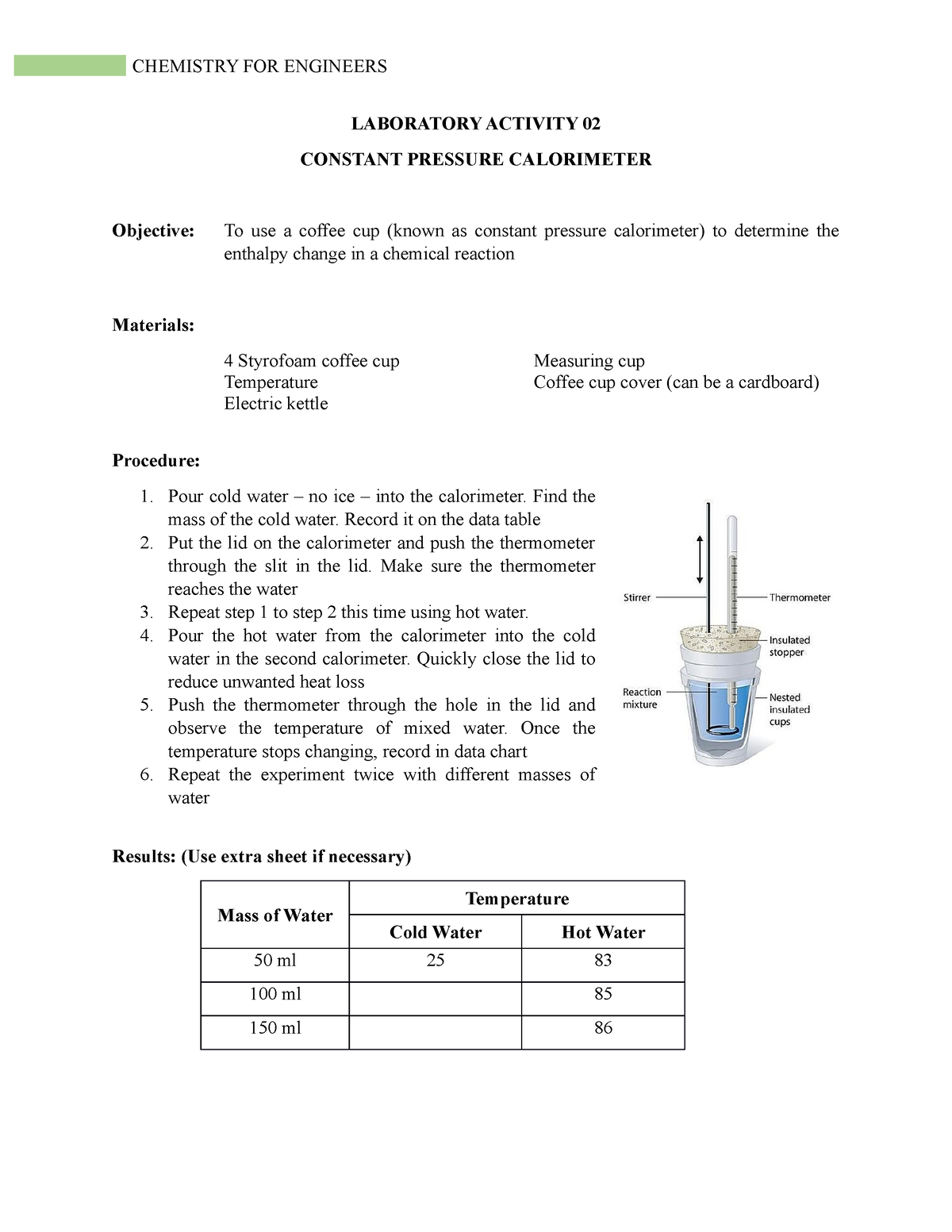
Lab02Calorimetry Lectures and activities LABORATORY ACTIVITY 02
Calorimetry Lab Activity C Measuring the latent heat of fusion of water, and measuring. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. As part of this lab, you will: The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. One way to do this is to place a sample of the. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimetry is the science of measuring heat flow. In this lab, you will do two classic calorimetry experiments: Calorimeters can be used to find a substance’s specific heat. Measuring the latent heat of fusion of water, and measuring. Measure the amount of heat involved in frostbite. The amount of heat that flows in or out of a. The specific heat of a substance is easily determined by comparing it to that of water.
From studylib.net
Lab 33 calorimetry lab Calorimetry Lab Activity C Calorimeters can be used to find a substance’s specific heat. The specific heat of a substance is easily determined by comparing it to that of water. Measure the amount of heat involved in frostbite. The amount of heat that flows in or out of a. As part of this lab, you will: One way to do this is to place. Calorimetry Lab Activity C.
From studylib.net
Calorimetry Worksheet Calorimetry Lab Activity C One way to do this is to place a sample of the. Calorimetry is the science of measuring heat flow. As part of this lab, you will: The amount of heat that flows in or out of a. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water. Calorimetry Lab Activity C.
From hazelbryanblogs.blogspot.com
Calorimetry Lab Gizmo Answers Activity C Chemistry Study Guide Calorimetry Lab Activity C When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat. As part of this lab, you will: Measuring the latent heat of fusion of water, and measuring. In this lab, you will do two classic calorimetry. Calorimetry Lab Activity C.
From all-game-guide.blogspot.com
Calorimetry Lab Gizmo Answers Activity C / Codominance Calorimetry Lab Activity C Measuring the latent heat of fusion of water, and measuring. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. In this lab, you will do two classic calorimetry experiments: Heat is defined as thermal energy flowing from an object at a higher temperature to one. Calorimetry Lab Activity C.
From www.studypool.com
SOLUTION Student exploration calorimetry lab Studypool Calorimetry Lab Activity C Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. One way to do this is to place a sample of the. The amount of heat that flows in or out of a. Calorimetry is the science of measuring heat flow. The specific heat of a substance is easily determined by. Calorimetry Lab Activity C.
From www.chegg.com
Mass (g) Chapter 10Calorimetry Worksheet Key Open Calorimetry Lab Activity C Measuring the latent heat of fusion of water, and measuring. Calorimetry is the science of measuring heat flow. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. One way to do this is to place a sample of the. Measure the amount of heat involved. Calorimetry Lab Activity C.
From klarxnzah.blob.core.windows.net
Calorimetry Experiment With Different Metals at David Lytton blog Calorimetry Lab Activity C Calorimeters can be used to find a substance’s specific heat. The specific heat of a substance is easily determined by comparing it to that of water. Calorimetry is the science of measuring heat flow. In this lab, you will do two classic calorimetry experiments: When a hot object is placed in the calorimeter, heat energy is transferred from the object. Calorimetry Lab Activity C.
From topupgamecandycrush.blogspot.com
Calorimetry Lab Gizmo Answers Activity C Based on the graphs below Calorimetry Lab Activity C The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. As part of this lab, you will: Measuring the latent heat of fusion of water, and measuring. Calorimeters can be used to find a substance’s specific heat. In this lab, you will do two classic calorimetry experiments: One way. Calorimetry Lab Activity C.
From studylib.net
Chemistry 1 Unit 2 Calorimetry Lab Calorimetry Lab Activity C Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. In this lab, you will do two classic calorimetry experiments: Calorimetry is the science of measuring heat flow. Calorimeters can be used to find a substance’s specific heat. Measure the amount of heat involved in frostbite. The specific heat of a. Calorimetry Lab Activity C.
From sfsgyfbhpf.blogspot.com
Calorimetry Lab Gizmo Answers Activity C Calorimetrylabse Pdf Daniel Calorimetry Lab Activity C The specific heat of a substance is easily determined by comparing it to that of water. As part of this lab, you will: In this lab, you will do two classic calorimetry experiments: One way to do this is to place a sample of the. When a hot object is placed in the calorimeter, heat energy is transferred from the. Calorimetry Lab Activity C.
From www.studocu.com
05+Solution+Calorimetry+PreLab Solution Calorimetry PreLab Calorimetry Lab Activity C Measuring the latent heat of fusion of water, and measuring. Measure the amount of heat involved in frostbite. Calorimetry is the science of measuring heat flow. The amount of heat that flows in or out of a. The specific heat of a substance is easily determined by comparing it to that of water. The calorimeter constant ccal calorimetry is the. Calorimetry Lab Activity C.
From get1millionvisitor.blogspot.com
Calorimetry Lab Gizmo Answers Activity C A 5 2 1 ½ B 5 2 0 ½ C 5 1 2 Calorimetry Lab Activity C One way to do this is to place a sample of the. Calorimetry is the science of measuring heat flow. The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. Measure the amount of heat involved in frostbite. Calorimeters can be used to find a substance’s specific heat. In. Calorimetry Lab Activity C.
From www.studypool.com
SOLUTION Calorimetry Lab Studypool Calorimetry Lab Activity C Calorimeters can be used to find a substance’s specific heat. The amount of heat that flows in or out of a. Measure the amount of heat involved in frostbite. One way to do this is to place a sample of the. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water. Calorimetry Lab Activity C.
From www.chegg.com
Solved Chapter 6 Calorimetry Worksheet Key Open the Calorimetry Lab Activity C In this lab, you will do two classic calorimetry experiments: Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. The specific heat of a substance is easily determined by comparing it to that of water. Measure the amount of heat involved in frostbite. As part of this lab, you will:. Calorimetry Lab Activity C.
From paja-k.blogspot.com
Calorimetry Lab Gizmo Answers Activity C / Calorimetry Computer Calorimetry Lab Activity C In this lab, you will do two classic calorimetry experiments: Calorimeters can be used to find a substance’s specific heat. Measure the amount of heat involved in frostbite. As part of this lab, you will: One way to do this is to place a sample of the. Measuring the latent heat of fusion of water, and measuring. Calorimetry is the. Calorimetry Lab Activity C.
From afashionlista.blogspot.com
Answer Key Calorimetry Lab Gizmo Answers Activity C / Explore Learning Calorimetry Lab Activity C Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Calorimetry is the science of measuring heat flow. The amount of heat that flows in or out of a. Measure the amount of heat involved in frostbite. The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat. Calorimetry Lab Activity C.
From www.docsity.com
This is a chem lab Calorimetry Activity Study Guides, Projects Calorimetry Lab Activity C The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. Calorimeters can be used to find a substance’s specific heat. Measuring the latent heat of fusion of water, and measuring. Calorimetry is the science of measuring heat flow. When a hot object is placed in the calorimeter, heat energy. Calorimetry Lab Activity C.
From studylib.net
Calorimetry Worksheet Calorimetry Lab Activity C When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Measure the amount of heat involved in frostbite. One way to do this is to place a. Calorimetry Lab Activity C.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Lab Activity C Measuring the latent heat of fusion of water, and measuring. As part of this lab, you will: Calorimeters can be used to find a substance’s specific heat. Calorimetry is the science of measuring heat flow. In this lab, you will do two classic calorimetry experiments: Measure the amount of heat involved in frostbite. One way to do this is to. Calorimetry Lab Activity C.
From www.coursehero.com
[Solved] Food Calorimetry Lab Activity I need help answering the d ata Calorimetry Lab Activity C Calorimetry is the science of measuring heat flow. In this lab, you will do two classic calorimetry experiments: One way to do this is to place a sample of the. Calorimeters can be used to find a substance’s specific heat. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. When. Calorimetry Lab Activity C.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Calorimetry Lab Activity C The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. One way to do this is to place a sample of the. The amount of. Calorimetry Lab Activity C.
From afashionlista.blogspot.com
Answer Key Calorimetry Lab Gizmo Answers Activity C / Explore Learning Calorimetry Lab Activity C Measuring the latent heat of fusion of water, and measuring. In this lab, you will do two classic calorimetry experiments: As part of this lab, you will: The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. Calorimetry is the science of measuring heat flow. One way to do. Calorimetry Lab Activity C.
From losform.athensmutualaid.net
Student Exploration Calorimetry Lab Gizmo Answer Key losformathens Calorimetry Lab Activity C The specific heat of a substance is easily determined by comparing it to that of water. The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. As part of this lab, you will: One way to do this is to place a sample of the. Heat is defined as. Calorimetry Lab Activity C.
From bruntonclithapping.blogspot.com
Answer Key Calorimetry Lab Gizmo Answers Activity C Student Calorimetry Lab Activity C As part of this lab, you will: Calorimeters can be used to find a substance’s specific heat. One way to do this is to place a sample of the. The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. Measuring the latent heat of fusion of water, and measuring.. Calorimetry Lab Activity C.
From www.scribd.com
Lab 4 Calorimetry Lab PDF Temperature Heat Capacity Calorimetry Lab Activity C As part of this lab, you will: Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. In this lab, you will do two classic calorimetry experiments: When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up.. Calorimetry Lab Activity C.
From www.coursehero.com
[Solved] Food Calorimetry Lab Activity I need help answering the d ata Calorimetry Lab Activity C The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. In this lab, you will do two classic calorimetry experiments: The amount of heat that flows in or out of a. Calorimeters can be used to find a substance’s specific heat. As part of this lab, you will: Heat. Calorimetry Lab Activity C.
From purchasingyamaha88keys.blogspot.com
Calorimetry Lab Gizmo Answers Activity C Flavio Tonon It Follow the Calorimetry Lab Activity C Measuring the latent heat of fusion of water, and measuring. As part of this lab, you will: When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimetry is the science of measuring heat flow. In this lab, you will do two classic calorimetry experiments: Heat. Calorimetry Lab Activity C.
From www.coursehero.com
[Solved] Food Calorimetry Lab Activity I need help answering the d ata Calorimetry Lab Activity C As part of this lab, you will: Calorimeters can be used to find a substance’s specific heat. The amount of heat that flows in or out of a. Calorimetry is the science of measuring heat flow. Measure the amount of heat involved in frostbite. One way to do this is to place a sample of the. Heat is defined as. Calorimetry Lab Activity C.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry Lab Activity C The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. As part of this lab, you will: In this lab, you will do two classic calorimetry experiments: Measure the amount of heat involved in frostbite. When a hot object is placed in the calorimeter, heat energy is transferred from. Calorimetry Lab Activity C.
From www.studocu.com
Lab02Calorimetry Lectures and activities LABORATORY ACTIVITY 02 Calorimetry Lab Activity C In this lab, you will do two classic calorimetry experiments: Calorimetry is the science of measuring heat flow. One way to do this is to place a sample of the. Measure the amount of heat involved in frostbite. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water. Calorimetry Lab Activity C.
From www.studocu.com
4 1 Fundamentals of Calorimetry Lab Report Dr Z Edits The Calorimetry Lab Activity C As part of this lab, you will: When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimetry is the science of measuring heat flow. The specific heat of a substance is easily determined by comparing it to that of water. Measure the amount of heat. Calorimetry Lab Activity C.
From www.studypool.com
SOLUTION Calorimetry lab gizmo all answers correct Studypool Calorimetry Lab Activity C One way to do this is to place a sample of the. Measure the amount of heat involved in frostbite. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. The calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or. Calorimetry Lab Activity C.
From www.chegg.com
Solved CALORIMETRY HEAT CAPACITY OF A CALORIMETER Calorimetry Lab Activity C Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Measure the amount of heat involved in frostbite. As part of this lab, you will: One way to do this is to place a sample of the. In this lab, you will do two classic calorimetry experiments: The amount of heat. Calorimetry Lab Activity C.
From www.studocu.com
Calorimetry Gizmo Student Exploration Calorimetry Lab Gizmo Warmup Calorimetry Lab Activity C Measure the amount of heat involved in frostbite. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat. One way to do this is to place a sample of the. Measuring the latent heat of fusion. Calorimetry Lab Activity C.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Lab Activity C Calorimeters can be used to find a substance’s specific heat. Measuring the latent heat of fusion of water, and measuring. Measure the amount of heat involved in frostbite. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower. Calorimetry is the science of measuring heat flow. The calorimeter constant ccal calorimetry. Calorimetry Lab Activity C.