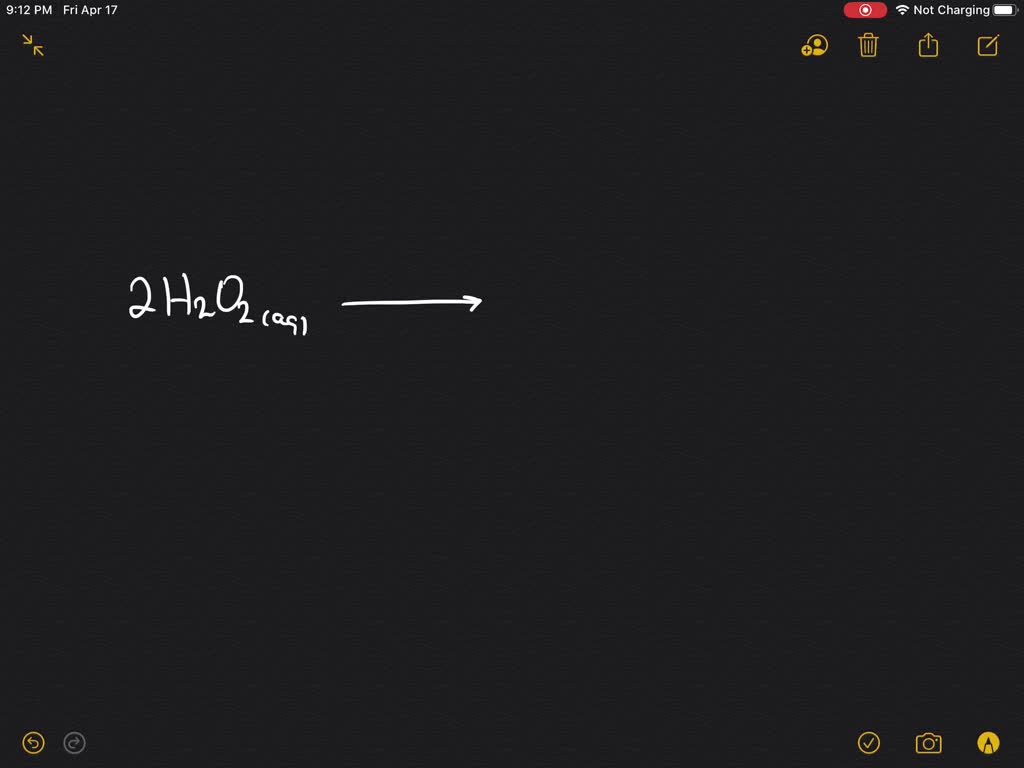Manganese Iv Oxide Decomposition . Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. To address it, an electrochemical activation. In this article, we present an overview on the thermocatalytic reaction of. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling.
from www.numerade.com
Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. In this article, we present an overview on the thermocatalytic reaction of. It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. To address it, an electrochemical activation.
SOLVEDA common demonstration in chemistry courses involves adding a
Manganese Iv Oxide Decomposition Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. To address it, an electrochemical activation. It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling. In this article, we present an overview on the thermocatalytic reaction of.
From studylib.net
RR5Does the amount of the catalyst manganese (IV) oxide change Manganese Iv Oxide Decomposition In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. [42] developed a procedure for the oxidative. Manganese Iv Oxide Decomposition.
From www.befr.ebay.be
Dioxyde de manganèse Oxyde de manganèse (IV) Dioxomanganèse Peroxyde Manganese Iv Oxide Decomposition [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. To address it, an electrochemical activation. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas. Manganese Iv Oxide Decomposition.
From www.youtube.com
of hydrogen peroxide with Manganese dioxide YouTube Manganese Iv Oxide Decomposition In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling. In this article, we present an overview on the thermocatalytic. Manganese Iv Oxide Decomposition.
From www.youtube.com
How to Write the Formula for Manganese (IV) oxide (or Manganese dioxide Manganese Iv Oxide Decomposition [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. In this article, we present an overview on the thermocatalytic reaction of. Manganese (iv) oxide (mno 2). Manganese Iv Oxide Decomposition.
From www.numerade.com
SOLVEDA common demonstration in chemistry courses involves adding a Manganese Iv Oxide Decomposition In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling. To address it, an electrochemical activation. In this article, we. Manganese Iv Oxide Decomposition.
From segudanggambar748.blogspot.com
Manganese Iv Oxide Formula / Manganese Oxide Chemical Formula Manganese Iv Oxide Decomposition [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. To address it, an electrochemical activation. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. In this article, we present an overview on. Manganese Iv Oxide Decomposition.
From pubs.acs.org
Mechanisms of Manganese Oxide Electrocatalysts Degradation during Manganese Iv Oxide Decomposition Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. To address it, an electrochemical activation. In this article, we present an overview on the thermocatalytic reaction of. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. In this article, we present an overview on the thermocatalytic reaction of. Manganese Iv Oxide Decomposition.
From www.tessshebaylo.com
Balanced Equation For The Breakdown Of Hydrogen Peroxide By Manganese Manganese Iv Oxide Decomposition Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. To address it, an electrochemical activation. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas. Manganese Iv Oxide Decomposition.
From www.researchgate.net
(PDF) SYNTHESIS AND CATALYTIC ACTIVITY OF DISPERSED MANGANESE(IV Manganese Iv Oxide Decomposition In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. To address it, an electrochemical activation. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. In this article, we present an overview on the thermocatalytic reaction of. It is revealed that mn. Manganese Iv Oxide Decomposition.
From www.semanticscholar.org
Figure 1 from Thermal of KClO4 Catalyzed with Pure and Manganese Iv Oxide Decomposition [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. In this article, we present an overview on the thermocatalytic reaction of. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. To address it, an electrochemical activation. In this article, we present an overview on. Manganese Iv Oxide Decomposition.
From www.youtube.com
How to Write the Formula for Manganese (IV) oxide YouTube Manganese Iv Oxide Decomposition In this article, we present an overview on the thermocatalytic reaction of. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling. In this article, we present. Manganese Iv Oxide Decomposition.
From www.youtube.com
Catalytic of hydrogen peroxide using Manganese(IV)oxide Manganese Iv Oxide Decomposition Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide. Manganese Iv Oxide Decomposition.
From www.studypool.com
SOLUTION Investigation into the effect of increasing the concentration Manganese Iv Oxide Decomposition To address it, an electrochemical activation. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. In this article, we present an overview on. Manganese Iv Oxide Decomposition.
From mdpi.com
Nanomaterials Free FullText Thermocatalytic Behavior of Manganese Manganese Iv Oxide Decomposition Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. To address it, an electrochemical activation. In this article, we present an overview on the thermocatalytic reaction of. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse. Manganese Iv Oxide Decomposition.
From www.numerade.com
SOLVEDA common demonstration in chemistry courses involves adding a Manganese Iv Oxide Decomposition In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. It is revealed that mn (iii) oxidation. Manganese Iv Oxide Decomposition.
From www.gauthmath.com
Solved 9. Oxygen is produced by the of hydrogen peroxide Manganese Iv Oxide Decomposition To address it, an electrochemical activation. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. It is revealed that mn (iii) oxidation to. Manganese Iv Oxide Decomposition.
From www.youtube.com
How to write chemical formula of Manganese IV OxideManganese IV Oxide Manganese Iv Oxide Decomposition In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. To address it, an electrochemical activation. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. [42]. Manganese Iv Oxide Decomposition.
From www.slideserve.com
PPT Naming Multivalent Compounds PowerPoint Presentation, free Manganese Iv Oxide Decomposition In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. In. Manganese Iv Oxide Decomposition.
From www.911metallurgist.com
Extraction of Manganese by Leaching Manganese Iv Oxide Decomposition Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. Manganese. Manganese Iv Oxide Decomposition.
From lab.honeywell.com
Manganese(IV) oxide 217646 Honeywell Research Chemicals Manganese Iv Oxide Decomposition [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. Manganese oxides have great potential. Manganese Iv Oxide Decomposition.
From www.alamy.com
Catalysed Adding manganese (IV) oxide (MnO2) to hydrogen Manganese Iv Oxide Decomposition Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. To address it, an electrochemical activation. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. In this article, we present an overview on the thermocatalytic reaction of. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as. Manganese Iv Oxide Decomposition.
From pubs.acs.org
Understanding the Role of Manganese Dioxide in the Oxidation of Manganese Iv Oxide Decomposition Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. [42] developed a procedure for the oxidative. Manganese Iv Oxide Decomposition.
From www.scribd.com
Comparing the Catalytic Effects of Manganese(IV) Oxide, Lead(IV) Oxide Manganese Iv Oxide Decomposition It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound. Manganese Iv Oxide Decomposition.
From segudanggambar748.blogspot.com
Manganese Iv Oxide Formula / Manganese Oxide Chemical Formula Manganese Iv Oxide Decomposition Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. In. Manganese Iv Oxide Decomposition.
From journals.asm.org
Manganese Oxide Biomineralization Provides Protection against Nitrite Manganese Iv Oxide Decomposition [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. In this article, we present an overview on the thermocatalytic reaction of. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. To address it, an electrochemical activation. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse. Manganese Iv Oxide Decomposition.
From pixels.com
Manganese (iv) Oxide Photograph by Andrew Lambert Photography Pixels Manganese Iv Oxide Decomposition Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. To address it, an electrochemical. Manganese Iv Oxide Decomposition.
From www.researchgate.net
Structures of various Mn oxides (MnOx). Shown are examples of tunnelled Manganese Iv Oxide Decomposition Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. To address it, an electrochemical activation. In this article, we present an overview on the thermocatalytic reaction of. It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling. Manganese oxides have great potential. Manganese Iv Oxide Decomposition.
From www.studypool.com
SOLUTION Investigation into the effect of increasing the concentration Manganese Iv Oxide Decomposition To address it, an electrochemical activation. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. In this article, we present an overview on the thermocatalytic reaction of. It is revealed that mn (iii) oxidation to. Manganese Iv Oxide Decomposition.
From www.youtube.com
of hydrogen peroxide with manganese dioxide YouTube Manganese Iv Oxide Decomposition Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. To address it, an electrochemical activation. It is revealed that mn (iii) oxidation to. Manganese Iv Oxide Decomposition.
From www.mdpi.com
Materials Free FullText Fabrication of SinglePhase Manganese Manganese Iv Oxide Decomposition It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. In this article, we present an overview on the thermocatalytic reaction of. [42] developed a procedure for the oxidative decomposition of chrome ores,. Manganese Iv Oxide Decomposition.
From www.youtube.com
Manganese Oxide Nanoparticle Synthesis By Thermal l Manganese Iv Oxide Decomposition In this article, we present an overview on the thermocatalytic reaction of. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. To address it, an electrochemical activation. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. It is revealed that mn (iii) oxidation to mn (iv) is the. Manganese Iv Oxide Decomposition.
From slideplayer.com
Catalyst. ppt video online download Manganese Iv Oxide Decomposition Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. To address it, an electrochemical activation. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. It. Manganese Iv Oxide Decomposition.
From www.scienceabc.com
Manganese Oxide Chemical Formula, Properties And Uses » ScienceABC Manganese Iv Oxide Decomposition To address it, an electrochemical activation. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse range of industrial catalytic. It is revealed that mn (iii) oxidation to mn (iv) is the primary cause of mn/mn 3 o 4 activity loss during orr/oer potential cycling. [42] developed a procedure for the oxidative decomposition of chrome ores, as well. Manganese Iv Oxide Decomposition.
From www.numerade.com
An aqueous solution of hydrogen peroxide very slowly at room Manganese Iv Oxide Decomposition In this article, we present an overview on the thermocatalytic reaction of. [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. To address it, an electrochemical activation. Manganese (iv) oxide (mno 2) is a tetravalent oxide compound of manganese. Manganese oxides have great potential as selective heterogeneous catalysts toward a diverse. Manganese Iv Oxide Decomposition.
From www.alamy.com
Full labelled diagram of the laboratory preparation of oxygen from Manganese Iv Oxide Decomposition [42] developed a procedure for the oxidative decomposition of chrome ores, as well as chromium (iii) oxide by. In this article, we present an overview on the thermocatalytic reaction of. In this article, we present an overview on the thermocatalytic reaction of hydrogen peroxide (h 2 o 2 ) gas on a manganese (iv) oxide. Manganese (iv) oxide (mno 2). Manganese Iv Oxide Decomposition.