Ester Resonance Structures . An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Identify the general structure for an ester. Name esters according to the iupac system. Write the mechanism of acidic ester hydrolysis. Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Use common names to name esters. Write the mechanism of alkaline ester hydrolysis. Identify the structure of an unknown ester, given the products of its hydrolysis. The forward reaction is a hydrolysis; Ester hydrolysis is common in biological chemistry,.
from www.chegg.com
As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Identify the structure of an unknown ester, given the products of its hydrolysis. Write the mechanism of acidic ester hydrolysis. An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Identify the general structure for an ester. Ester hydrolysis is common in biological chemistry,. Write the mechanism of alkaline ester hydrolysis. Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. The forward reaction is a hydrolysis; Name esters according to the iupac system.
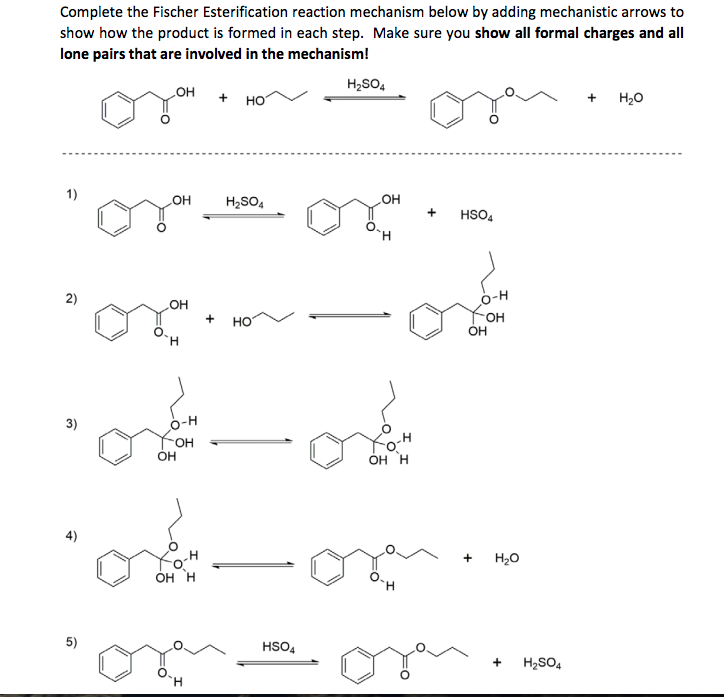
Solved Complete the Fischer Esterification reaction
Ester Resonance Structures The forward reaction is a hydrolysis; Identify the general structure for an ester. Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. Ester hydrolysis is common in biological chemistry,. As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Identify the structure of an unknown ester, given the products of its hydrolysis. Use common names to name esters. Write the mechanism of alkaline ester hydrolysis. Write the mechanism of acidic ester hydrolysis. The forward reaction is a hydrolysis; An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Name esters according to the iupac system.
From www.masterorganicchemistry.com
The Malonic Ester and Acetoacetic Ester Synthesis Master Organic Ester Resonance Structures Ester hydrolysis is common in biological chemistry,. The forward reaction is a hydrolysis; Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. Write the mechanism of acidic ester hydrolysis. Identify the structure of an unknown ester, given the products of its hydrolysis.. Ester Resonance Structures.
From www.masterorganicchemistry.com
Conjugation and Resonance — Master Organic Chemistry Ester Resonance Structures The forward reaction is a hydrolysis; Use common names to name esters. Identify the general structure for an ester. As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Ester hydrolysis is common in biological chemistry,. Write the mechanism of acidic ester hydrolysis. Write the mechanism of alkaline ester hydrolysis.. Ester Resonance Structures.
From chemistryscore.com
Resonance Structures Learn Chemistry Online ChemistryScore Ester Resonance Structures The forward reaction is a hydrolysis; As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Use common names to name esters. Write the mechanism of acidic ester hydrolysis. Identify the general structure for an ester. Write the mechanism of alkaline ester hydrolysis. Identify the structure of an unknown ester,. Ester Resonance Structures.
From www.online-sciences.com
Physical and chemical properties of Esters, Esterification reaction and Ester Resonance Structures Ester hydrolysis is common in biological chemistry,. Use common names to name esters. Write the mechanism of alkaline ester hydrolysis. Identify the structure of an unknown ester, given the products of its hydrolysis. Write the mechanism of acidic ester hydrolysis. As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:.. Ester Resonance Structures.
From www.slideserve.com
PPT Chapter 11 Reactions of Alcohols PowerPoint Presentation ID151694 Ester Resonance Structures The forward reaction is a hydrolysis; Write the mechanism of alkaline ester hydrolysis. Name esters according to the iupac system. Write the mechanism of acidic ester hydrolysis. Identify the structure of an unknown ester, given the products of its hydrolysis. Ester hydrolysis is common in biological chemistry,. Draw three resonance contributors of methyl acetate (an ester with the structure ch. Ester Resonance Structures.
From www.slideserve.com
PPT The Esters PowerPoint Presentation, free download ID1828351 Ester Resonance Structures Identify the general structure for an ester. Ester hydrolysis is common in biological chemistry,. An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Identify the structure of an unknown ester, given the products of its hydrolysis. Draw three resonance contributors of methyl acetate. Ester Resonance Structures.
From www.chemistrysteps.com
Naming Esters Chemistry Steps Ester Resonance Structures Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. The forward reaction is a hydrolysis; Use. Ester Resonance Structures.
From openpress.usask.ca
5.5. Resonance Introduction to Organic Chemistry Ester Resonance Structures Identify the general structure for an ester. Identify the structure of an unknown ester, given the products of its hydrolysis. Use common names to name esters. The forward reaction is a hydrolysis; Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. Name. Ester Resonance Structures.
From www.coursehero.com
[Solved] 4. The resonance structure of an ester is shown below. (a Ester Resonance Structures Identify the structure of an unknown ester, given the products of its hydrolysis. Ester hydrolysis is common in biological chemistry,. Write the mechanism of alkaline ester hydrolysis. As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Use common names to name esters. Name esters according to the iupac system.. Ester Resonance Structures.
From www.aceorganicchem.com
What is resonance? [7 rules to master it] Organic chemistry help Ester Resonance Structures Use common names to name esters. Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. The forward reaction is a hydrolysis; Ester hydrolysis is common in biological chemistry,. Name esters according to the iupac system. Write the mechanism of acidic ester hydrolysis.. Ester Resonance Structures.
From www.solutionspile.com
[Solved] What are the resonance structures of the enolate Ester Resonance Structures The forward reaction is a hydrolysis; Identify the general structure for an ester. Identify the structure of an unknown ester, given the products of its hydrolysis. Use common names to name esters. Write the mechanism of acidic ester hydrolysis. Name esters according to the iupac system. As we’ve seen in previous articles, four key factors that determine the importance of. Ester Resonance Structures.
From www.chegg.com
Chemistry Archive April 02, 2017 Ester Resonance Structures Identify the general structure for an ester. Ester hydrolysis is common in biological chemistry,. Identify the structure of an unknown ester, given the products of its hydrolysis. Write the mechanism of alkaline ester hydrolysis. Use common names to name esters. Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according. Ester Resonance Structures.
From socratic.org
What is the esterification reaction equation of benzyl alcohol and Ester Resonance Structures Name esters according to the iupac system. Identify the general structure for an ester. The forward reaction is a hydrolysis; Ester hydrolysis is common in biological chemistry,. An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Identify the structure of an unknown ester,. Ester Resonance Structures.
From www.chemistrysteps.com
Naming Esters Chemistry Steps Ester Resonance Structures Name esters according to the iupac system. As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Identify the structure of an unknown ester, given the products of its hydrolysis. Write the mechanism of acidic ester hydrolysis. Identify the general structure for an ester. Write the mechanism of alkaline ester. Ester Resonance Structures.
From www.masterorganicchemistry.com
Fischer Esterification Carboxylic Acid to Ester Under Acidic Ester Resonance Structures Name esters according to the iupac system. Write the mechanism of alkaline ester hydrolysis. Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. Use common names to name esters. As we’ve seen in previous articles, four key factors that determine the importance. Ester Resonance Structures.
From www.reddit.com
Chemistry Teacher AMA r/GCSE Ester Resonance Structures Use common names to name esters. Write the mechanism of acidic ester hydrolysis. As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding.. Ester Resonance Structures.
From studylib.net
Carboxylic Acids and Esters Ester Resonance Structures As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Identify the structure of an unknown ester, given the products of its hydrolysis. Write the mechanism of acidic ester hydrolysis. Name esters according to the iupac system. Use common names to name esters. Identify the general structure for an ester.. Ester Resonance Structures.
From wisc.pb.unizin.org
Day 12 Intermolecular Forces; Functional Groups Chemistry 109, Fall 2020 Ester Resonance Structures Identify the general structure for an ester. Use common names to name esters. An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Write the mechanism of alkaline ester hydrolysis. Write the mechanism of acidic ester hydrolysis. Identify the structure of an unknown ester,. Ester Resonance Structures.
From chem.libretexts.org
12.4 Esters Chemistry LibreTexts Ester Resonance Structures As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Write the mechanism of alkaline ester hydrolysis. An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Use common names to name esters. Write. Ester Resonance Structures.
From www.chegg.com
Solved 13. Draw out the resonance structures which show how Ester Resonance Structures An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. The forward reaction is a hydrolysis; Identify the general structure for an ester. Write the mechanism of alkaline ester hydrolysis. Identify the structure of an unknown ester, given the products of its hydrolysis. Name. Ester Resonance Structures.
From mavink.com
Ester Lewis Structure Ester Resonance Structures As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Write the mechanism of alkaline ester hydrolysis. The forward reaction is a hydrolysis; An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Ester. Ester Resonance Structures.
From www.chegg.com
Solved Complete the Fischer Esterification reaction Ester Resonance Structures Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Write the mechanism of alkaline ester hydrolysis.. Ester Resonance Structures.
From www.clutchprep.com
Acetoacetic Ester Synthesis Organic Chemistry Video Clutch Prep Ester Resonance Structures Identify the general structure for an ester. Use common names to name esters. Write the mechanism of acidic ester hydrolysis. An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Write the mechanism of alkaline ester hydrolysis. Name esters according to the iupac system.. Ester Resonance Structures.
From www.slideserve.com
PPT Esters PowerPoint Presentation, free download ID2192073 Ester Resonance Structures Identify the structure of an unknown ester, given the products of its hydrolysis. Identify the general structure for an ester. Use common names to name esters. Write the mechanism of acidic ester hydrolysis. Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding.. Ester Resonance Structures.
From courses.lumenlearning.com
6.2. Resonance Organic Chemistry 1 An open textbook Ester Resonance Structures Write the mechanism of acidic ester hydrolysis. Write the mechanism of alkaline ester hydrolysis. As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Use common names to name esters. The forward reaction is a hydrolysis; Ester hydrolysis is common in biological chemistry,. Identify the structure of an unknown ester,. Ester Resonance Structures.
From www.masterorganicchemistry.com
Conjugation And Resonance In Organic Chemistry Ester Resonance Structures Write the mechanism of acidic ester hydrolysis. Use common names to name esters. Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. Ester hydrolysis is common in biological chemistry,. Identify the general structure for an ester. The forward reaction is a hydrolysis;. Ester Resonance Structures.
From www.chegg.com
Solved Provide both resonance structures of the enolate Ester Resonance Structures The forward reaction is a hydrolysis; As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Ester hydrolysis is common in biological chemistry,. Identify the general structure for an ester. Use common names to name esters. An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded. Ester Resonance Structures.
From www.numerade.com
SOLVEDDraw possible structures for an ester that has a molecular ion Ester Resonance Structures Ester hydrolysis is common in biological chemistry,. Identify the structure of an unknown ester, given the products of its hydrolysis. Identify the general structure for an ester. Write the mechanism of alkaline ester hydrolysis. Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the. Ester Resonance Structures.
From chem.libretexts.org
20.6 Ester Chemistry Chemistry LibreTexts Ester Resonance Structures Identify the general structure for an ester. Name esters according to the iupac system. Identify the structure of an unknown ester, given the products of its hydrolysis. Ester hydrolysis is common in biological chemistry,. Use common names to name esters. Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according. Ester Resonance Structures.
From mungfali.com
Ester Molecule Structure Ester Resonance Structures Write the mechanism of acidic ester hydrolysis. The forward reaction is a hydrolysis; Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. Write the mechanism of alkaline ester hydrolysis. Name esters according to the iupac system. Ester hydrolysis is common in biological. Ester Resonance Structures.
From www.masterorganicchemistry.com
Conjugation And Resonance In Organic Chemistry Ester Resonance Structures Ester hydrolysis is common in biological chemistry,. The forward reaction is a hydrolysis; As we’ve seen in previous articles, four key factors that determine the importance of resonance structures in organic chemistry are:. Identify the structure of an unknown ester, given the products of its hydrolysis. Use common names to name esters. Write the mechanism of acidic ester hydrolysis. Draw. Ester Resonance Structures.
From courses.lumenlearning.com
15.6 Esters Structures and Names The Basics of General, Organic, and Ester Resonance Structures An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Write the mechanism of alkaline ester hydrolysis. Name esters according to the iupac system. Use common names to name esters. Identify the structure of an unknown ester, given the products of its hydrolysis. Write. Ester Resonance Structures.
From chemdictionary.org
Ester Definition, Examples And Facts Chemistry Dictionary Ester Resonance Structures Write the mechanism of alkaline ester hydrolysis. Write the mechanism of acidic ester hydrolysis. Identify the general structure for an ester. An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Ester hydrolysis is common in biological chemistry,. Name esters according to the iupac. Ester Resonance Structures.
From openpress.usask.ca
5.5. Resonance Introduction to Organic Chemistry Ester Resonance Structures Write the mechanism of alkaline ester hydrolysis. Use common names to name esters. An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. The forward reaction is a hydrolysis; Ester hydrolysis is common in biological chemistry,. Identify the structure of an unknown ester, given. Ester Resonance Structures.
From www.chegg.com
Solved Draw the resonance structures for the betaketo ester Ester Resonance Structures Draw three resonance contributors of methyl acetate (an ester with the structure ch 3 cooch 3), and order them according to their relative importance to the bonding. The forward reaction is a hydrolysis; An esterfunctional group contains a carbonyl group with a second oxygen atom single bonded to the carbonyl carbon and also single bonded to another carbon atom. Write. Ester Resonance Structures.