Catalysts Examples Names . Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Many catalysts are transition metals. You will also find a description of one. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. You will also find a description of one example of. Enzymes are naturally occurring catalysts responsible for many essential biochemical. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids;
from www.slideshare.net
You will also find a description of one example of. Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Many catalysts are transition metals. You will also find a description of one.
Catalyst
Catalysts Examples Names Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Enzymes are naturally occurring catalysts responsible for many essential biochemical. Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. Many catalysts are transition metals. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. You will also find a description of one. You will also find a description of one example of. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids;
From www.researchgate.net
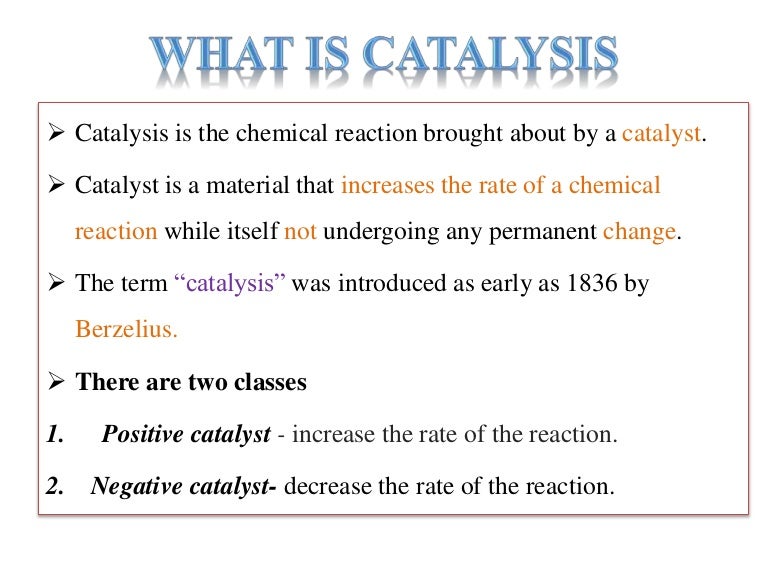
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalysts Examples Names This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. You will also find a description of one. For example, platinum is the catalyst in an automobile catalytic converter that. Catalysts Examples Names.
From joilxpqmt.blob.core.windows.net
Chemical Catalysis Examples at Howard Wade blog Catalysts Examples Names You will also find a description of one. Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of. Catalysts Examples Names.
From exomdmjjp.blob.core.windows.net
Examples Of Catalysts Bbc Bitesize at Jacqueline Epp blog Catalysts Examples Names Many catalysts are transition metals. Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. Enzymes are naturally occurring catalysts responsible for many essential biochemical. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. The most effective catalyst of all. Catalysts Examples Names.
From www.researchgate.net
2. List of Catalysts Tested and Corresponding Labels Download Table Catalysts Examples Names A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. For example, platinum is the catalyst in an automobile catalytic converter. Catalysts Examples Names.
From www.youtube.com
Identifying catalysts in a reaction YouTube Catalysts Examples Names Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. This page looks at the the different types of catalyst (heterogeneous. Catalysts Examples Names.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysts Examples Names Many catalysts are transition metals. Enzymes are naturally occurring catalysts responsible for many essential biochemical. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. For example, platinum is the catalyst in an automobile catalytic converter that turns. Catalysts Examples Names.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation Catalysts Examples Names Many catalysts are transition metals. Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. You will also find a description of one example of. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a substance that. Catalysts Examples Names.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind Catalysts Examples Names You will also find a description of one example of. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Many catalysts are transition metals. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used. Catalysts Examples Names.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysts Examples Names For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; A catalyst. Catalysts Examples Names.
From www.slideserve.com
PPT Catalysts in Organic Synthesis PowerPoint Presentation, free Catalysts Examples Names For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. You will also find a description of one. Many catalysts are transition metals. This page looks at. Catalysts Examples Names.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation Catalysts Examples Names A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. This page looks at the the different types of catalyst (heterogeneous. Catalysts Examples Names.
From inspiritvr.com
CBSE Class 12 Chemistry Chapter 5 Revision Notes Inspirit Catalysts Examples Names This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. You will also find a description of one. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Catalyst, in chemistry,. Catalysts Examples Names.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalysts Examples Names This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. You will also find a description of one. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. Adding a drop. Catalysts Examples Names.
From joiryfmry.blob.core.windows.net
Enzymes As Catalysts Examples at Mayra McCue blog Catalysts Examples Names You will also find a description of one example of. You will also find a description of one. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy. Catalysts Examples Names.
From ceriyrra.blob.core.windows.net
Catalyst Examples Class 10 at Timothy Hunt blog Catalysts Examples Names This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction. Catalysts Examples Names.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID4503154 Catalysts Examples Names This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. You will also find a description of one. You will also find a description of one example of. For example,. Catalysts Examples Names.
From www.slideserve.com
PPT 23.5 Features of homogeneous catalysis PowerPoint Presentation Catalysts Examples Names The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; You will also find a description of one example of. Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and. Catalysts Examples Names.
From www.researchgate.net
Commonly Used Types of Catalysts and Their Range of Use Download Catalysts Examples Names Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. You will also find a description of one. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without. Catalysts Examples Names.
From www.mdpi.com
Catalysts Free FullText Green Chemistry in Organic Synthesis Catalysts Examples Names For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. Many catalysts are transition metals. A catalyst is a substance that. Catalysts Examples Names.
From www.slideshare.net
Catalysis Catalysts Examples Names Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. You will also find a. Catalysts Examples Names.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste Catalysts Examples Names Many catalysts are transition metals. Enzymes are naturally occurring catalysts responsible for many essential biochemical. For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; Catalyst, in chemistry, any substance that increases the rate of a. Catalysts Examples Names.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalysts Examples Names This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Many catalysts are transition metals. Enzymes are naturally occurring catalysts responsible for many essential biochemical. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used. Catalysts Examples Names.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalysts Examples Names You will also find a description of one. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. You will also find a description of one example of. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Adding a. Catalysts Examples Names.
From www.researchgate.net
Classification and summary of different catalysts. Download Catalysts Examples Names For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. You will also find a description of one example of. Enzymes are naturally occurring catalysts responsible for many essential biochemical. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. Catalysts Examples Names.
From slideplayer.com
Biological catalysts Enzymes IGCSE Biology. ppt download Catalysts Examples Names Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many essential biochemical. Many catalysts are transition metals. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. This page looks at. Catalysts Examples Names.
From www.slideserve.com
PPT Topic 06 6.2a Collision theory 6.2B Maxwell Catalysts Examples Names Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; You will also find a description of one. Many catalysts are. Catalysts Examples Names.
From www.slideshare.net
Heterogeneous catalysisFundamentals Catalysts Examples Names Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. For example, platinum is the. Catalysts Examples Names.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2823635 Catalysts Examples Names Many catalysts are transition metals. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. You will also find a description of one. This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how. Catalysts Examples Names.
From www.slideshare.net
Catalyst Catalysts Examples Names You will also find a description of one example of. Many catalysts are transition metals. Enzymes are naturally occurring catalysts responsible for many essential biochemical. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. This. Catalysts Examples Names.
From www.slideserve.com
PPT Topic 06 6.2a Collision theory 6.2B Maxwell Catalysts Examples Names Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. Enzymes are naturally occurring catalysts responsible for many essential biochemical. You will also find a description of one example of. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction.. Catalysts Examples Names.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Examples Names The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; This page looks at the the different types of catalyst (heterogeneous and homogeneous) with examples of each kind, and explanations of how they work. Many catalysts are transition metals. You will also find a description of one. Adding a drop of blood to a. Catalysts Examples Names.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica Catalysts Examples Names Many catalysts are transition metals. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used. Catalysts Examples Names.
From ceriyrra.blob.core.windows.net
Catalyst Examples Class 10 at Timothy Hunt blog Catalysts Examples Names You will also find a description of one. Enzymes are naturally occurring catalysts responsible for many essential biochemical. Many catalysts are transition metals. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used. Catalysts Examples Names.
From zymvol.com
All you need to know about enzymes Zymvol Catalysts Examples Names Enzymes are naturally occurring catalysts responsible for many essential biochemical. You will also find a description of one example of. You will also find a description of one. Adding a drop of blood to a solution of 30% hydrogen peroxide induces a vigorous. The most effective catalyst of all is the enzyme catalase, present in blood and intracellular fluids; This. Catalysts Examples Names.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free Catalysts Examples Names A catalyst is a substance that increases the rate of a chemical reaction by lowering the activation energy without being used up in the reaction. For example, platinum is the catalyst in an automobile catalytic converter that turns carbon monoxide into carbon dioxide. You will also find a description of one example of. Enzymes are naturally occurring catalysts responsible for. Catalysts Examples Names.