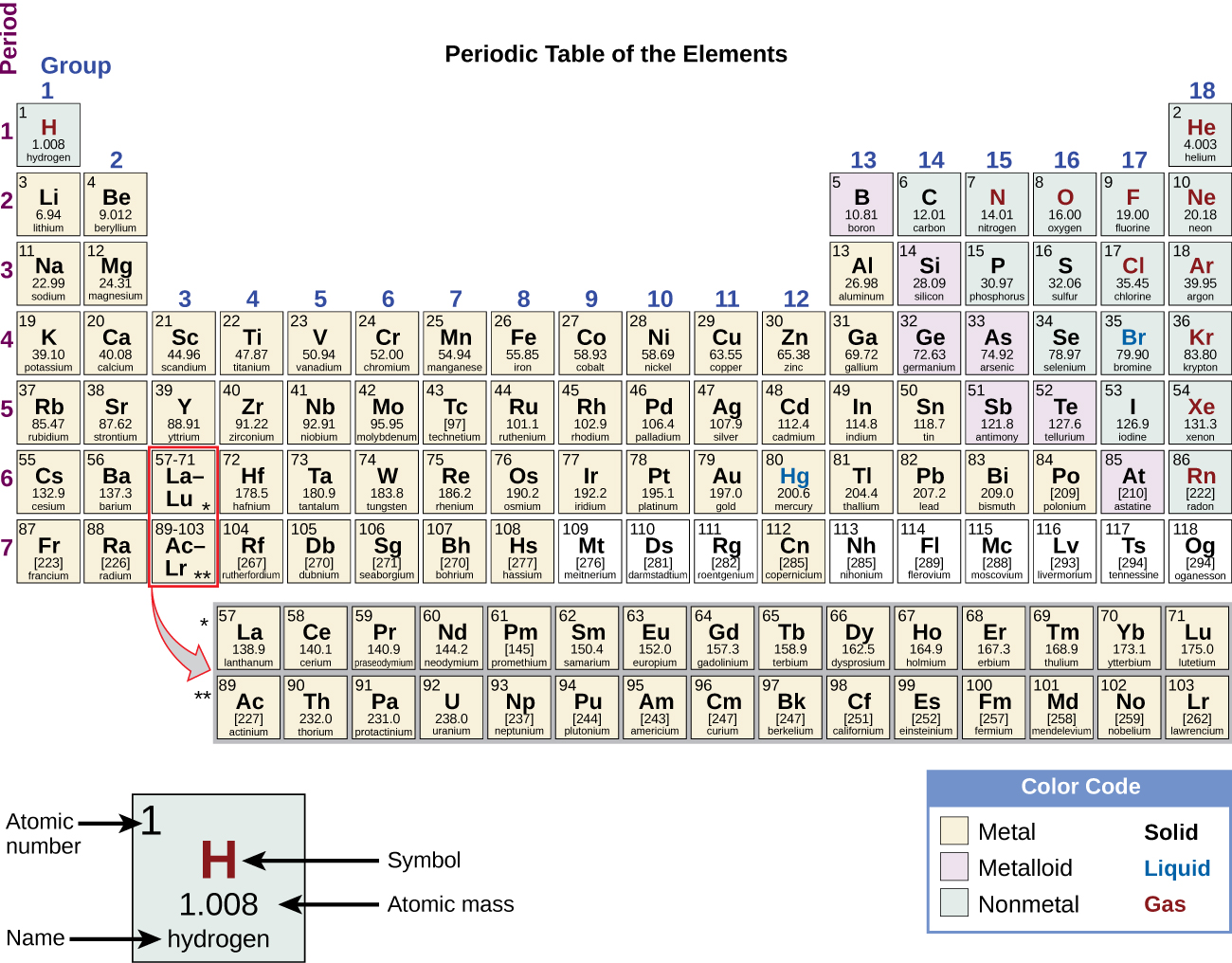
Do Elements In A Period Have Similar Properties . The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear in the same vertical. Elements within a period share the same number of electron shells and the same highest unexcited electron energy level. The valence electrons mainly determine the chemical properties of the elements. Elements within a period display periodic table trends,. The elements in the same group have similar chemical. On the periodic table, elements that have similar properties are in the same groups (vertical). Elements in the same period may have different chemical properties, but they share the same number of electron shells. All the members of a family of elements have the. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). In summary, groups represent elements with similar properties due to their valence. From left to right, the atomic number (z). On the periodic table, elements that have similar properties are in the same groups (vertical).
from pressbooks.openedmb.ca
The elements in the same group have similar chemical. On the periodic table, elements that have similar properties are in the same groups (vertical). Elements within a period share the same number of electron shells and the same highest unexcited electron energy level. From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). In summary, groups represent elements with similar properties due to their valence. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. Elements in the same period may have different chemical properties, but they share the same number of electron shells. From left to right, the atomic number (z). Elements within a period display periodic table trends,. On the periodic table, elements that have similar properties are in the same groups (vertical).
The Periodic Table Chemistry and the Environment
Do Elements In A Period Have Similar Properties On the periodic table, elements that have similar properties are in the same groups (vertical). On the periodic table, elements that have similar properties are in the same groups (vertical). All the members of a family of elements have the. In summary, groups represent elements with similar properties due to their valence. On the periodic table, elements that have similar properties are in the same groups (vertical). The elements in the same group have similar chemical. From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). Elements within a period display periodic table trends,. Elements within a period share the same number of electron shells and the same highest unexcited electron energy level. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. From left to right, the atomic number (z). The valence electrons mainly determine the chemical properties of the elements. Elements in the same period may have different chemical properties, but they share the same number of electron shells. The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear in the same vertical.
From mungfali.com
Which Two Elements Have Similar Characteristics Do Elements In A Period Have Similar Properties The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. Elements in the same period may have different chemical properties, but they share the same number of electron shells. From left to right, the atomic number (z). On the periodic table, elements that have similar properties are in the same groups (vertical).. Do Elements In A Period Have Similar Properties.
From brainly.in
The diagram shows two early attempts at the periodic table. In both Do Elements In A Period Have Similar Properties From left to right, the atomic number (z). Elements in the same period may have different chemical properties, but they share the same number of electron shells. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. In summary, groups represent elements with similar properties due to their valence. On the periodic. Do Elements In A Period Have Similar Properties.
From megan-yersbloganderson.blogspot.com
Which Two Elements Have Similar Properties Do Elements In A Period Have Similar Properties The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. Elements within a period display periodic table trends,. Elements within a period share the same number of electron shells and the same highest unexcited electron energy level. All the members of a family of elements have the. On the periodic table, elements. Do Elements In A Period Have Similar Properties.
From www.britannica.com
Chemical compound Trends in the chemical properties of the elements Do Elements In A Period Have Similar Properties The valence electrons mainly determine the chemical properties of the elements. The elements in the same group have similar chemical. From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). From left to right, the atomic number (z). The vertical columns on the periodic table are called groups or families because. Do Elements In A Period Have Similar Properties.
From www.slideserve.com
PPT The Periodic Table of Elements PowerPoint Presentation, free Do Elements In A Period Have Similar Properties From left to right, the atomic number (z). The elements in the same group have similar chemical. The valence electrons mainly determine the chemical properties of the elements. Elements within a period display periodic table trends,. In summary, groups represent elements with similar properties due to their valence. The vertical columns on the periodic table are called groups or families. Do Elements In A Period Have Similar Properties.
From chemassist.blogspot.com
ChemAssist Groups of the Periodic Table Do Elements In A Period Have Similar Properties The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. In summary, groups represent elements with similar properties due to their valence. On the periodic table, elements that have similar properties are in the same groups (vertical). The valence electrons mainly determine the chemical properties of the elements. Elements in the same. Do Elements In A Period Have Similar Properties.
From periodictableguide.com
Periods in Periodic table Explained! (With 17 Period Names) Do Elements In A Period Have Similar Properties Elements within a period share the same number of electron shells and the same highest unexcited electron energy level. In summary, groups represent elements with similar properties due to their valence. Elements within a period display periodic table trends,. From left to right, the atomic number (z). On the periodic table, elements that have similar properties are in the same. Do Elements In A Period Have Similar Properties.
From www.britannica.com
Periodic table Elements, Properties, Periodicity Britannica Do Elements In A Period Have Similar Properties The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear in the same vertical. Elements within a period display periodic table trends,. On the periodic table, elements that have similar properties are in the same groups (vertical). Elements within a period share the same number of electron shells. Do Elements In A Period Have Similar Properties.
From www.haikudeck.com
Chemistry Periodic Table by James Rowland Do Elements In A Period Have Similar Properties The elements in the same group have similar chemical. Elements within a period share the same number of electron shells and the same highest unexcited electron energy level. All the members of a family of elements have the. Elements in the same period may have different chemical properties, but they share the same number of electron shells. The vertical columns. Do Elements In A Period Have Similar Properties.
From orangesciences.blogspot.com
LSS Sec 2 Periodic Table Do Elements In A Period Have Similar Properties Elements within a period display periodic table trends,. The valence electrons mainly determine the chemical properties of the elements. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear in. Do Elements In A Period Have Similar Properties.
From courses.lumenlearning.com
Reading The Periodic Table of Elements Biology (Early Release) Do Elements In A Period Have Similar Properties The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. The valence electrons mainly determine the chemical properties of the elements. The elements in the same group have similar chemical. The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear. Do Elements In A Period Have Similar Properties.
From chemwiki.ucdavis.edu
Periodic Properties of the Elements Chemwiki Do Elements In A Period Have Similar Properties The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear in the same vertical. On the periodic table, elements that have similar properties are in the same groups (vertical). The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. The. Do Elements In A Period Have Similar Properties.
From byjus.com
Do elements in the same group have similar physical properties or not? Do Elements In A Period Have Similar Properties Elements in the same period may have different chemical properties, but they share the same number of electron shells. From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear. Do Elements In A Period Have Similar Properties.
From powerpointban.web.fc2.com
Why do elements in the same group have similar chemical properties Do Elements In A Period Have Similar Properties From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). Elements within a period display periodic table trends,. From left to right, the atomic number (z). In summary, groups represent elements with similar properties due to their valence. On the periodic table, elements that have similar properties are in the same. Do Elements In A Period Have Similar Properties.
From www.amnh.org
How to Read the Periodic Table AMNH Do Elements In A Period Have Similar Properties On the periodic table, elements that have similar properties are in the same groups (vertical). From left to right, the atomic number (z). In summary, groups represent elements with similar properties due to their valence. All the members of a family of elements have the. From left to right, the atomic number (z) of the elements increases from one period. Do Elements In A Period Have Similar Properties.
From mavink.com
Periodic Table Of Elements Names List Do Elements In A Period Have Similar Properties From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). Elements in the same period may have different chemical properties, but they share the same number of electron shells. The valence electrons mainly determine the chemical properties of the elements. The periodic table is an arrangement of the elements in order. Do Elements In A Period Have Similar Properties.
From dashamlav.com
Periodic Table Groups Names and Properties Do Elements In A Period Have Similar Properties Elements within a period display periodic table trends,. The elements in the same group have similar chemical. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. From left to right, the atomic number (z). Elements in the same period may have different chemical properties, but they share the same number of. Do Elements In A Period Have Similar Properties.
From pressbooks.openedmb.ca
The Periodic Table Chemistry and the Environment Do Elements In A Period Have Similar Properties All the members of a family of elements have the. The elements in the same group have similar chemical. Elements in the same period may have different chemical properties, but they share the same number of electron shells. On the periodic table, elements that have similar properties are in the same groups (vertical). On the periodic table, elements that have. Do Elements In A Period Have Similar Properties.
From studylib.net
The Periodic Table Do Elements In A Period Have Similar Properties Elements within a period share the same number of electron shells and the same highest unexcited electron energy level. From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). The valence electrons mainly determine the chemical properties of the elements. The elements in the same group have similar chemical. In summary,. Do Elements In A Period Have Similar Properties.
From www.periodictableprintable.com
How Many Electrons Does 1a Periodic Table 2023 Periodic Table Printable Do Elements In A Period Have Similar Properties From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). Elements within a period share the same number of electron shells and the same highest unexcited electron energy level. The elements in the same group have similar chemical. On the periodic table, elements that have similar properties are in the same. Do Elements In A Period Have Similar Properties.
From adelyn-has-mahoney.blogspot.com
Which Elements Have the Most Similar Chemical Properties Adelynhas Do Elements In A Period Have Similar Properties On the periodic table, elements that have similar properties are in the same groups (vertical). Elements in the same period may have different chemical properties, but they share the same number of electron shells. From left to right, the atomic number (z). On the periodic table, elements that have similar properties are in the same groups (vertical). The valence electrons. Do Elements In A Period Have Similar Properties.
From sites.google.com
C2 The Periodic Table Kingshill Science Do Elements In A Period Have Similar Properties In summary, groups represent elements with similar properties due to their valence. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. Elements within a period share the same number of electron shells and the same highest unexcited electron energy level. Elements within a period display periodic table trends,. From left to. Do Elements In A Period Have Similar Properties.
From thechemistrynotes.com
Chemical Properties Of Period 3 Elements of Periodic Table Do Elements In A Period Have Similar Properties On the periodic table, elements that have similar properties are in the same groups (vertical). The elements in the same group have similar chemical. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. On the periodic table, elements that have similar properties are in the same groups (vertical). Elements within a. Do Elements In A Period Have Similar Properties.
From www.thoughtco.com
Element Families of the Periodic Table Do Elements In A Period Have Similar Properties Elements within a period display periodic table trends,. All the members of a family of elements have the. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. Elements in the same period may have different chemical properties, but they share the same number of electron shells. From left to right, the. Do Elements In A Period Have Similar Properties.
From www.slideserve.com
PPT Why do elements in the same group/family have similar properties Do Elements In A Period Have Similar Properties The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear in the same vertical. On the periodic table, elements that have similar properties are in the same groups (vertical). The elements in the same group have similar chemical. From left to right, the atomic number (z) of the. Do Elements In A Period Have Similar Properties.
From www.researchgate.net
Periodic table Elements display similar properties at regular periods Do Elements In A Period Have Similar Properties Elements within a period display periodic table trends,. From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). All the members of a family of elements have the. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. The elements in the same. Do Elements In A Period Have Similar Properties.
From www.thoughtco.com
The Periodic Properties of the Elements Do Elements In A Period Have Similar Properties From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). Elements within a period share the same number of electron shells and the same highest unexcited electron energy level. From left to right, the atomic number (z). All the members of a family of elements have the. In summary, groups represent. Do Elements In A Period Have Similar Properties.
From typeost.com
Similar Chemical Properties To Magnesium TypeOst Do Elements In A Period Have Similar Properties In summary, groups represent elements with similar properties due to their valence. From left to right, the atomic number (z). From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). On the periodic table, elements that have similar properties are in the same groups (vertical). On the periodic table, elements that. Do Elements In A Period Have Similar Properties.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Do Elements In A Period Have Similar Properties The elements in the same group have similar chemical. All the members of a family of elements have the. On the periodic table, elements that have similar properties are in the same groups (vertical). Elements within a period share the same number of electron shells and the same highest unexcited electron energy level. Elements within a period display periodic table. Do Elements In A Period Have Similar Properties.
From www.researchgate.net
Periodic table Elements display similar properties at regular periods Do Elements In A Period Have Similar Properties From left to right, the atomic number (z). The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear in the same vertical. Elements within a period display periodic table trends,. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior.. Do Elements In A Period Have Similar Properties.
From sciencenotes.org
Element Families on the Periodic Table Do Elements In A Period Have Similar Properties On the periodic table, elements that have similar properties are in the same groups (vertical). Elements within a period display periodic table trends,. The elements in the same group have similar chemical. From left to right, the atomic number (z) of the elements increases from one period to the next (horizontal). The vertical columns on the periodic table are called. Do Elements In A Period Have Similar Properties.
From powerpointban.web.fc2.com
Why do elements in the same group have similar chemical properties Do Elements In A Period Have Similar Properties The valence electrons mainly determine the chemical properties of the elements. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. From left to right, the atomic number (z). On the periodic table, elements that have similar properties are in the same groups (vertical). In summary, groups represent elements with similar properties. Do Elements In A Period Have Similar Properties.
From brainly.com
Using the periodic table, explain which pair of the following gases Do Elements In A Period Have Similar Properties The elements in the same group have similar chemical. The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear in the same vertical. The valence electrons mainly determine the chemical properties of the elements. In summary, groups represent elements with similar properties due to their valence. From left. Do Elements In A Period Have Similar Properties.
From www.thoughtco.com
Properties of Periodic Table of Element Groups Do Elements In A Period Have Similar Properties In summary, groups represent elements with similar properties due to their valence. All the members of a family of elements have the. Elements in the same period may have different chemical properties, but they share the same number of electron shells. The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with. Do Elements In A Period Have Similar Properties.
From spmscience.blog.onlinetuition.com.my
4.4 Classification of Elements in the Period Table SPM Science Do Elements In A Period Have Similar Properties Elements within a period display periodic table trends,. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. All the members of a family of elements have the. The periodic table is an arrangement of the elements in order of their atomic numbers so that elements with similar properties appear in the. Do Elements In A Period Have Similar Properties.