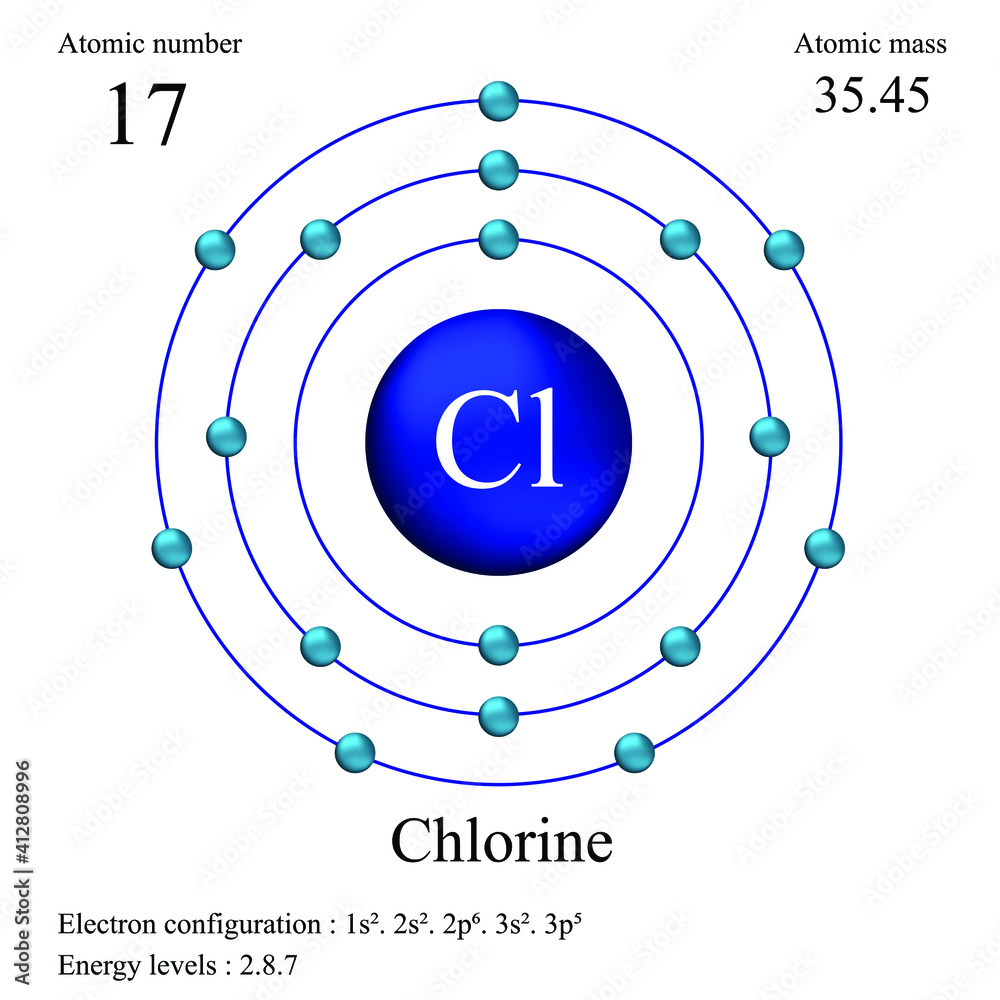
Chlorine Single Atom . [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Chlorine (cl) has an atomic mass of 17. The atomic mass or relative isotopic mass refers to. If a single value of the atomic mass is needed, use 35.4527. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. The atomic mass is the mass of an atom. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. It has an atomic weight of 35.450 and a mass. Atomic mass of chlorine is 35.453 u.
from stock.adobe.com
Atomic mass of chlorine is 35.453 u. It has an atomic weight of 35.450 and a mass. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. The atomic mass or relative isotopic mass refers to. If a single value of the atomic mass is needed, use 35.4527. The atomic mass is the mass of an atom. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Chlorine (cl) has an atomic mass of 17. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery:
Chlorine atomic structure has atomic number, atomic mass, electron
Chlorine Single Atom It has an atomic weight of 35.450 and a mass. Atomic mass of chlorine is 35.453 u. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. If a single value of the atomic mass is needed, use 35.4527. The atomic mass or relative isotopic mass refers to. Chlorine (cl) has an atomic mass of 17. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. The atomic mass is the mass of an atom. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). It has an atomic weight of 35.450 and a mass. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Chlorine Single Atom Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. The atomic mass or relative isotopic mass refers to. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in. Chlorine Single Atom.
From www.shutterstock.com
Bohr Model Chlorine Atom Electron Structure vetor stock (livre de Chlorine Single Atom Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. It has an atomic weight of 35.450 and a mass. Atomic. Chlorine Single Atom.
From www.sciencephoto.com
Chlorine molecules Stock Image A602/0069 Science Photo Library Chlorine Single Atom It has an atomic weight of 35.450 and a mass. The atomic mass or relative isotopic mass refers to. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Chlorine (cl) has an atomic mass of 17. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl). Chlorine Single Atom.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Single Atom Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. Chlorine Single Atom.
From manualfixdercombustion.z13.web.core.windows.net
Chlorine Electron Dot Diagram Chlorine Single Atom [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Find out about its chemical and physical properties,. Chlorine Single Atom.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Single Atom It has an atomic weight of 35.450 and a mass. Chlorine (cl) has an atomic mass of 17. If a single value of the atomic mass is needed, use 35.4527. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: Chlorine, like many of the other halogens, can form interhalogen compounds (examples. Chlorine Single Atom.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Chlorine Single Atom Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. The atomic mass or relative isotopic mass refers to. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl. Chlorine Single Atom.
From utedzz.blogspot.com
Periodic Table Chlorine Atom Periodic Table Timeline Chlorine Single Atom The atomic mass or relative isotopic mass refers to. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Find out about its chemical and physical properties, states, energy, electrons, oxidation and. Chlorine Single Atom.
From ar.inspiredpencil.com
3d Chlorine Atom Model Chlorine Single Atom The atomic mass is the mass of an atom. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: It has an atomic weight of 35.450 and a mass. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting. Chlorine Single Atom.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Single Atom Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Atomic mass of chlorine is 35.453 u. If a single value of the atomic mass is needed, use 35.4527. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: It has an atomic weight of 35.450 and a. Chlorine Single Atom.
From www.animalia-life.club
Chlorine Element Periodic Table Chlorine Single Atom The atomic mass or relative isotopic mass refers to. It has an atomic weight of 35.450 and a mass. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Chlorine (cl) has an. Chlorine Single Atom.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Single Atom The atomic mass is the mass of an atom. Atomic mass of chlorine is 35.453 u. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). If a single value of the atomic mass is needed, use 35.4527. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p. Chlorine Single Atom.
From www.shalom-education.com
Covalent Bonding GCSE Chemistry Revision Chlorine Single Atom [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine (cl) has an atomic mass of 17.. Chlorine Single Atom.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Single Atom It has an atomic weight of 35.450 and a mass. Chlorine (cl) has an atomic mass of 17. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). The atomic mass is the mass of an atom. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5. Chlorine Single Atom.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Single Atom Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. The atomic mass or relative isotopic mass refers to. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl. Chlorine Single Atom.
From www.alamy.com
Chlorine atom hires stock photography and images Alamy Chlorine Single Atom Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). If a single value of the atomic mass is needed, use 35.4527. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: Atomic mass of chlorine is 35.453 u. Chlorine (cl) has an atomic mass. Chlorine Single Atom.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Single Atom It has an atomic weight of 35.450 and a mass. The atomic mass is the mass of an atom. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). The atomic mass or relative isotopic mass refers to. Chlorine (cl) has an atomic mass of 17. Chlorine is the 17th element in the. Chlorine Single Atom.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Single Atom Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. If a single value of the atomic mass is needed, use 35.4527. The atomic mass is the mass of an atom. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Chlorine (cl). Chlorine Single Atom.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Chlorine Single Atom Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Chlorine (cl) has an atomic mass of 17. The atomic mass or relative isotopic mass refers to. The atomic mass is the mass. Chlorine Single Atom.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Single Atom [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. It has an atomic weight of 35.450 and a mass. Atomic mass of chlorine is 35.453 u. The atomic mass or relative. Chlorine Single Atom.
From www.dreamstime.com
An Atom of Chlorine Diagram Stock Vector Illustration of structure Chlorine Single Atom If a single value of the atomic mass is needed, use 35.4527. It has an atomic weight of 35.450 and a mass. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction,. Chlorine Single Atom.
From www.alamy.com
Molecular Model of Chlorine (Cl2) Molecule. Vector Illustration Stock Chlorine Single Atom Chlorine (cl) has an atomic mass of 17. The atomic mass or relative isotopic mass refers to. It has an atomic weight of 35.450 and a mass. If a single value of the atomic mass is needed, use 35.4527. Atomic mass of chlorine is 35.453 u. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2. Chlorine Single Atom.
From sciencenotes.org
Chlorine Facts Chlorine Single Atom The atomic mass is the mass of an atom. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. It has an atomic weight of 35.450 and a mass. Atomic mass of chlorine is 35.453 u. The atomic mass or relative isotopic mass refers to. If a single value of the atomic mass is needed, use. Chlorine Single Atom.
From www.alamy.com
Cl Chlorine Chemical Element Periodic Table. Single element vector Chlorine Single Atom Atomic mass of chlorine is 35.453 u. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: Chlorine (cl) has an atomic mass of 17. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. It has an atomic weight of 35.450 and a mass. An atom of. Chlorine Single Atom.
From www.alamy.com
Chlorine Element High Resolution Stock Photography and Images Alamy Chlorine Single Atom The atomic mass or relative isotopic mass refers to. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. The atomic mass is the mass of an atom. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). If a single value of the atomic mass is needed, use. Chlorine Single Atom.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Chlorine Single Atom The atomic mass or relative isotopic mass refers to. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: An atom of sodium (na) donates one of its electrons to an atom. Chlorine Single Atom.
From mavink.com
Bohr Model Of Chlorine Chlorine Single Atom Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. If a single value of the atomic mass is needed, use 35.4527. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Atomic mass of chlorine is 35.453 u. [ne]3s 2 3p 5 (shorthand) or 1s. Chlorine Single Atom.
From www.dreamstime.com
Chlorine Atom, with Mass and Energy Levels. Stock Vector Illustration Chlorine Single Atom Atomic mass of chlorine is 35.453 u. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: It has an atomic weight of 35.450 and a mass. An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion. Chlorine Single Atom.
From www.alamy.com
Chlorine Element Stock Photos & Chlorine Element Stock Images Alamy Chlorine Single Atom An atom of sodium (na) donates one of its electrons to an atom of chlorine (cl) in a chemical reaction, and the resulting positive ion (na +) and negative ion (cl −) form a. Chlorine (cl) has an atomic mass of 17. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number. Chlorine Single Atom.
From www.alamy.com
Chlorine Element High Resolution Stock Photography and Images Alamy Chlorine Single Atom It has an atomic weight of 35.450 and a mass. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Chlorine (cl) has an atomic mass of 17. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). Find out about its chemical. Chlorine Single Atom.
From circuitdataboattrains.z14.web.core.windows.net
Chlorine Atom Diagram Chlorine Single Atom Atomic mass of chlorine is 35.453 u. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). The atomic mass or relative isotopic mass refers to. Find out about its chemical and physical properties, states,. Chlorine Single Atom.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Single Atom The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to. If a single value of the atomic mass is needed, use 35.4527. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2. Chlorine Single Atom.
From www.alamy.com
Cl Chlorine Chemical Element Periodic Table. Single vector illustration Chlorine Single Atom Atomic mass of chlorine is 35.453 u. If a single value of the atomic mass is needed, use 35.4527. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: The atomic mass. Chlorine Single Atom.
From stock.adobe.com
Chlorine atomic structure has atomic number, atomic mass, electron Chlorine Single Atom Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Atomic mass of chlorine is 35.453 u. It has an atomic weight of 35.450 and a mass. Chlorine, like many of the other halogens, can form interhalogen compounds (examples include brcl, icl, icl 2). The atomic mass or relative isotopic. Chlorine Single Atom.
From mavink.com
Chlorine Molecule Diagram Chlorine Single Atom Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. [ne]3s 2 3p 5 (shorthand) or 1s 2 2s 2 2p 6 3s 2 3p 5 (full) discovery: If a single value of the atomic mass. Chlorine Single Atom.