Can Salt Dissolve In Methanol . The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Maybe the liquid phase will remain. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. This is because the water is able to form. In this section we will apply. This is a highly complicated question, and the answer can't be derived by logic alone. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons).
from www.chegg.com
The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. Maybe the liquid phase will remain. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. In this section we will apply. This is because the water is able to form. This is a highly complicated question, and the answer can't be derived by logic alone.
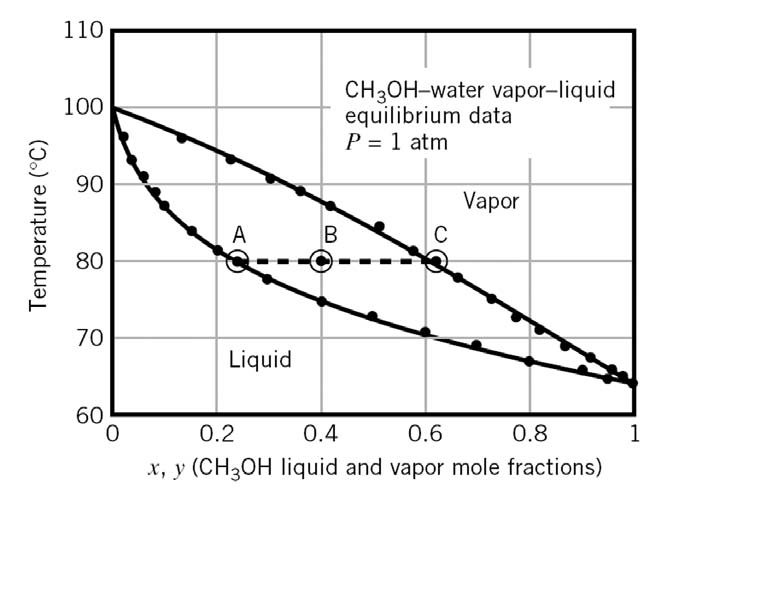
Solved A Txy Diagram For Methanol?water Mixtures At 1 Atm...
Can Salt Dissolve In Methanol Maybe the liquid phase will remain. This is because the water is able to form. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Maybe the liquid phase will remain. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. In this section we will apply. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is a highly complicated question, and the answer can't be derived by logic alone. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved.
From www.chegg.com
Solved A Txy Diagram For Methanol?water Mixtures At 1 Atm... Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). Using these rules we would. Can Salt Dissolve In Methanol.
From rdsoberliving.com
How Long is Meth in My System? Real Deal Meth Info Can Salt Dissolve In Methanol Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. In this section we will apply. This is a highly complicated question, and the answer can't be derived by logic alone. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is because the water is able. Can Salt Dissolve In Methanol.
From www.researchgate.net
Solubility comparison in methanol. Download Scientific Diagram Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is a highly complicated question, and the answer can't be derived by logic alone. Maybe the liquid phase will remain. This is because the water is able to form. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents. Can Salt Dissolve In Methanol.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Can Salt Dissolve In Methanol In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. In this section we will apply. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is a highly complicated question, and the answer can't be derived by logic alone. The solubilities of. Can Salt Dissolve In Methanol.
From www.acs.org
Simulations & Videos for Lesson 5.6 Does Temperature Affect Dissolving Can Salt Dissolve In Methanol Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). In this section we will apply. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. The. Can Salt Dissolve In Methanol.
From www.youtube.com
Solubility of salts and preparation of soluble salts YouTube Can Salt Dissolve In Methanol This is a highly complicated question, and the answer can't be derived by logic alone. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. The solubilities of sodium chloride, sodium bromide, and potassium. Can Salt Dissolve In Methanol.
From www.coursehero.com
[Solved] A student dissolves 14.g of methanol CH3OH in 200.mL of a Can Salt Dissolve In Methanol This is because the water is able to form. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Maybe the liquid phase will remain. In this section we will apply. Next, you try a series of increasingly. Can Salt Dissolve In Methanol.
From www.researchgate.net
Solubility of LiCl versus methanol content (salt free mole fractions Can Salt Dissolve In Methanol This is because the water is able to form. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. Maybe the liquid phase will remain. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Next, you try a series of increasingly large alcohol compounds, starting with methanol. Can Salt Dissolve In Methanol.
From www.numerade.com
SOLVEDAs the salt KNO3 dissolves in methanol, the number x(t) of grams Can Salt Dissolve In Methanol In this section we will apply. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is a highly complicated question, and the answer can't be derived by logic alone. Maybe the liquid phase will remain. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and. Can Salt Dissolve In Methanol.
From www.gbu-presnenskij.ru
Solubility Of Organic Compounds Chemistry Steps, 53 OFF Can Salt Dissolve In Methanol Maybe the liquid phase will remain. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. In. Can Salt Dissolve In Methanol.
From mungfali.com
Salt Solubility Chart Can Salt Dissolve In Methanol In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. Maybe the liquid phase will remain. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is because the water is able to form. Using these rules we would predict that insoluble salts. Can Salt Dissolve In Methanol.
From www.slideserve.com
PPT The Properties of Mixtures Solutions and Colloids PowerPoint Can Salt Dissolve In Methanol In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. This is because the water is able to form.. Can Salt Dissolve In Methanol.
From www.slideserve.com
PPT pH of Salts Solutions (Hydrolysis) PowerPoint Presentation, free Can Salt Dissolve In Methanol Maybe the liquid phase will remain. This is because the water is able to form. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. Using these rules we would. Can Salt Dissolve In Methanol.
From www.researchgate.net
Reaction of the salt 3 with methanol in the presence of Et3N and Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). Maybe the liquid phase will. Can Salt Dissolve In Methanol.
From eduvik.in
Matter in Our Surroundings Class 9 Notes Science Chapter 1 Eduvik Can Salt Dissolve In Methanol This is because the water is able to form. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. This is a highly complicated question, and the answer can't be derived by logic alone. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons).. Can Salt Dissolve In Methanol.
From www.pinterest.com.mx
Solubility Rules Chart for Chemistry Classroom Teaching chemistry Can Salt Dissolve In Methanol Maybe the liquid phase will remain. In this section we will apply. This is a highly complicated question, and the answer can't be derived by logic alone. This is because the water is able to form. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. The solubilities of sodium chloride, sodium bromide, and potassium. Can Salt Dissolve In Methanol.
From www.semanticscholar.org
Table 2 from Solubility of NaCl, NaBr, and KCl in Water, Methanol Can Salt Dissolve In Methanol In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). This is because the water is able to form. The solubilities of sodium chloride, sodium bromide, and potassium bromide in. Can Salt Dissolve In Methanol.
From www.reddit.com
Why does methanol byproduct and magnesium chloride salt form from this Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is because the water is able to form. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). This is a highly complicated question, and the answer can't be derived by. Can Salt Dissolve In Methanol.
From www.slideserve.com
PPT 4.4 Metallic bonding PowerPoint Presentation, free download ID Can Salt Dissolve In Methanol Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). In this section we will apply. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water,. Can Salt Dissolve In Methanol.
From www.johntalk.com
INFOGRAPHIC Methanol vs. Salt Brine JohnTalk Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). The solubilities of sodium chloride, sodium bromide,. Can Salt Dissolve In Methanol.
From www.semanticscholar.org
[PDF] Solubility of NaCl, NaBr, and KCl in Water, Methanol, Ethanol Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. This is because. Can Salt Dissolve In Methanol.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Can Salt Dissolve In Methanol This is because the water is able to form. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is a highly complicated question, and the answer can't be derived by logic alone. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol. Can Salt Dissolve In Methanol.
From pubs.acs.org
Journal of Chemical & Engineering Data Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is a highly complicated question, and the answer can't be derived by logic alone. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). The solubilities of sodium chloride, sodium bromide,. Can Salt Dissolve In Methanol.
From www.youtube.com
GCSE CHEMISTRY ACIDS AND BASES LESSON 19 salts solubility YouTube Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Maybe the liquid phase will remain. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Next,. Can Salt Dissolve In Methanol.
From www.toppr.com
Which pairs of the salts should have identical solubility in methanol? Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. In this section we will apply. This is a. Can Salt Dissolve In Methanol.
From www.wou.edu
CH150 Chapter 7 Solutions Chemistry Can Salt Dissolve In Methanol Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. This is a highly complicated question, and the answer can't be derived by logic alone. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. In table 1, the measured solubilities of the salts in water, methanol, and. Can Salt Dissolve In Methanol.
From www.semanticscholar.org
[PDF] Solubility of NaCl, NaBr, and KCl in Water, Methanol, Ethanol Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). This is a highly complicated question, and the answer can't be derived by logic alone. The solubilities of sodium chloride, sodium bromide,. Can Salt Dissolve In Methanol.
From www.youtube.com
Introduction to salts & solubility YouTube Can Salt Dissolve In Methanol Maybe the liquid phase will remain. This is because the water is able to form. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. In this section we will apply. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. Using these rules. Can Salt Dissolve In Methanol.
From www.youtube.com
Is CH3OH (Methanol) Soluble or Insoluble in Water? YouTube Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is because the water is able. Can Salt Dissolve In Methanol.
From mungfali.com
Salt Solubility Chart Can Salt Dissolve In Methanol This is because the water is able to form. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. In this section we will apply. Maybe the liquid phase will remain. The solubilities of. Can Salt Dissolve In Methanol.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Can Salt Dissolve In Methanol The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. In table 1, the measured solubilities of the salts in water, methanol, and ethanol at different temperatures are reported. This is because the water is able to form.. Can Salt Dissolve In Methanol.
From www.youtube.com
Methanol dissolve in water/ Solubility YouTube Can Salt Dissolve In Methanol Next, you try a series of increasingly large alcohol compounds, starting with methanol (1 carbon) and ending with octanol (8 carbons). In this section we will apply. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This. Can Salt Dissolve In Methanol.
From www.researchgate.net
Vapor pressure of methanol and ethanol as a function of temperature Can Salt Dissolve In Methanol In this section we will apply. Maybe the liquid phase will remain. This is a highly complicated question, and the answer can't be derived by logic alone. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is because the water is able to form. The solubilities of sodium chloride, sodium bromide,. Can Salt Dissolve In Methanol.
From www.chegg.com
Solved 23. As the salt KNO3 dissolves in methanol, the Can Salt Dissolve In Methanol This is because the water is able to form. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. This is a highly complicated question, and the answer can't be derived by logic alone. Using these rules we would predict that insoluble salts formed precipitates and soluble salts dissolved. In table 1, the. Can Salt Dissolve In Methanol.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets Can Salt Dissolve In Methanol This is a highly complicated question, and the answer can't be derived by logic alone. In this section we will apply. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. The solubilities of sodium chloride, sodium bromide, and potassium bromide in the solvents water, methanol, ethanol, and. Maybe the liquid phase will. Can Salt Dissolve In Methanol.