Standard Enthalpy Of Formation Of Potassium Bromide . 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including.
from www.slideserve.com
The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of.
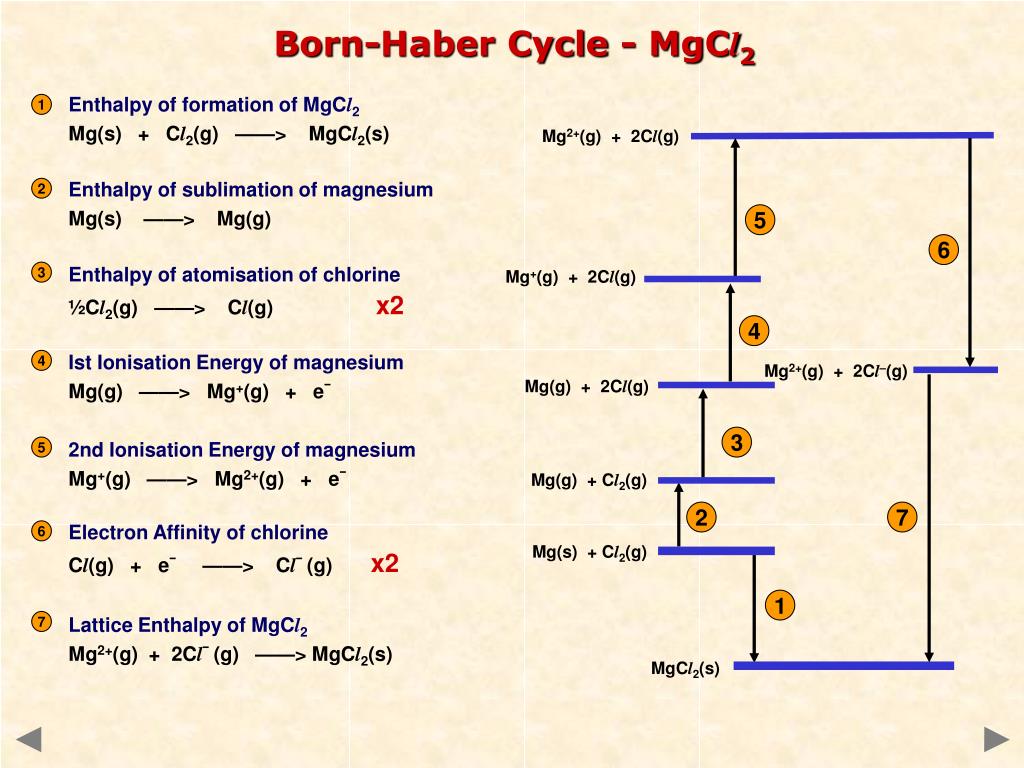
PPT Lattice enthalpy PowerPoint Presentation, free download ID6559902
Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a.
From exosbjzsg.blob.core.windows.net
Standard Enthalpy Of Formation Al2O3 at Lois Woodburn blog Standard Enthalpy Of Formation Of Potassium Bromide 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard enthalpy of formation (δh0f) of a compound is the change in. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 129 rows the standard gibbs free energy of formation (g f °). Standard Enthalpy Of Formation Of Potassium Bromide.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy. Standard Enthalpy Of Formation Of Potassium Bromide.
From mungfali.com
Table Of Enthalpy Of Formation Standard Enthalpy Of Formation Of Potassium Bromide Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation (δh0f) of a compound is the change in. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.numerade.com
SOLVED Write the chemical equation for the standard molar enthalpy of Standard Enthalpy Of Formation Of Potassium Bromide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.numerade.com
SOLVEDThe standard enthalpy of formation of gaseous molecular bromine Standard Enthalpy Of Formation Of Potassium Bromide Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 129 rows the standard gibbs free energy of formation (g f °) of a compound. Standard Enthalpy Of Formation Of Potassium Bromide.
From storage.googleapis.com
Standard Enthalpy Of Formation Sulfuric Acid Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. Standard enthalpy change of formation (data table) these tables include heat of. Standard Enthalpy Of Formation Of Potassium Bromide.
From shiken.ai
Born Haber Cycles Standard Enthalpy Of Formation Of Potassium Bromide 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 193 rows the standard enthalpy of formation is measured in units of. Standard Enthalpy Of Formation Of Potassium Bromide.
From studylib.net
Problem 1. The standard enthalpy of formation of KBr is Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standard enthalpy change of formation (data table) these tables include heat of. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.thesciencehive.co.uk
Lattice Enthalpy* — the science sauce Standard Enthalpy Of Formation Of Potassium Bromide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 193 rows the standard enthalpy of formation is measured in units of energy per amount of. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.pathwaystochemistry.com
Formation of Ionic Compounds The Born Haber Cycle Pathways to Chemistry Standard Enthalpy Of Formation Of Potassium Bromide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol). Standard Enthalpy Of Formation Of Potassium Bromide.
From www.youtube.com
Born Haber cycle for KBr YouTube Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.numerade.com
SOLVED Using the enthalpies of formation provided, determine the heat Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol). Standard Enthalpy Of Formation Of Potassium Bromide.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Standard enthalpy change of formation (data table) these tables include heat of. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 129 rows the standard gibbs free energy of formation (g f °). Standard Enthalpy Of Formation Of Potassium Bromide.
From www.toppr.com
(AU (B) 185 KJ Given, H.(g) + Br.(g) → 2HBr(g), AH, and standard Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation is defined as the change in enthalpy when one. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.sundarspine.com
Calculate The Heat Of Formation Of KCl From The Following, 44 OFF Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. Standand enthalpies of formation & standard entropies of common compounds. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.numerade.com
SOLVED Calculate the change internal energy Tor the thermal Standard Enthalpy Of Formation Of Potassium Bromide 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Standand enthalpies of formation & standard entropies of common compounds substance state. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Of Potassium Bromide 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 129 rows the standard gibbs free energy of formation (g f °). Standard Enthalpy Of Formation Of Potassium Bromide.
From byjus.com
KBr (potassium bromide) contains 32.9 Standard Enthalpy Of Formation Of Potassium Bromide Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. The standard enthalpy of formation is defined as the change in enthalpy when. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.chegg.com
Solved 10. Using the data below, calculate the lattice Standard Enthalpy Of Formation Of Potassium Bromide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 129 rows the standard gibbs free energy of formation (g f °) of a compound is. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.numerade.com
SOLVED a) Calculate the standard heat of the neutralization reaction Standard Enthalpy Of Formation Of Potassium Bromide 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation (δh0f) of a compound is the change. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Of Potassium Bromide 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 129 rows the standard gibbs free energy of formation (g f °) of a compound is. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Reaction for the Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standand enthalpies of formation & standard entropies of common compounds substance state. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.numerade.com
SOLVED Construct BornHaber cycle by adding the right processes and Standard Enthalpy Of Formation Of Potassium Bromide 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. The standard enthalpy of formation is defined as the change in enthalpy when. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5773812 Standard Enthalpy Of Formation Of Potassium Bromide 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 193 rows the standard enthalpy of formation is measured in units of. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standand enthalpies of formation & standard entropies of common compounds substance state ∆h f s (kjmol) (jmol·k) ag s 0 42.6 ag+. Standard enthalpy change of formation (data table) these tables include heat of formation. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.slideserve.com
PPT Lattice enthalpy PowerPoint Presentation, free download ID6559902 Standard Enthalpy Of Formation Of Potassium Bromide 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 129 rows the standard gibbs free energy of formation (g f °). Standard Enthalpy Of Formation Of Potassium Bromide.
From pt.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Of Potassium Bromide Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. 193 rows the standard enthalpy of formation is measured in units of energy per. Standard Enthalpy Of Formation Of Potassium Bromide.
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation Of Potassium Bromide 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. The standard enthalpy of formation (δh0f) of a compound is the change. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.numerade.com
SOLVED Potassium has a standard boiling point of 773 °C and a molar Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows the standard enthalpy of formation is measured in units of. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Of Potassium Bromide 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies. Standard Enthalpy Of Formation Of Potassium Bromide.
From schoolworkhelper.net
Standard Enthalpies of Formation SchoolWorkHelper Standard Enthalpy Of Formation Of Potassium Bromide 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. 193 rows the standard enthalpy of formation is measured in units of energy per amount of substance, usually stated in kilojoule per mole. Standand enthalpies of formation & standard entropies of common compounds substance state. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.numerade.com
SOLVED The enthalpy of solution for Potassium Bromide (KBr) is 19.9 Standard Enthalpy Of Formation Of Potassium Bromide The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 129 rows the standard gibbs free energy of formation (g f °) of a compound is. Standard Enthalpy Of Formation Of Potassium Bromide.
From www.researchgate.net
Enthalpies of dissolution of racemic tartaric acid in water at 298.15 K Standard Enthalpy Of Formation Of Potassium Bromide 129 rows the standard gibbs free energy of formation (g f °) of a compound is the change of gibbs free energy that accompanies the. The standard enthalpy of formation (δh0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a. 193 rows the standard enthalpy of formation is measured in units of. Standard Enthalpy Of Formation Of Potassium Bromide.