Chloride Ion Formula And Charge . the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. If given a formula like nacl or feo or fe 2 o 3 you need to be able to calculate the charge of the ions. determining ion charges from formula. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. sodium chloride, or table salt, is an ionic compound. Since the neutral halogen atom. The precise pattern depends on the compound. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. the chloride ion has a single, negative charge. So that the charges are balanced and it is neutral overall. Let’s take a look at how it is formed.
from www.chem.ucla.edu
So that the charges are balanced and it is neutral overall. Let’s take a look at how it is formed. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. the chloride ion has a single, negative charge. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. If given a formula like nacl or feo or fe 2 o 3 you need to be able to calculate the charge of the ions. Since the neutral halogen atom. The precise pattern depends on the compound. determining ion charges from formula. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride.
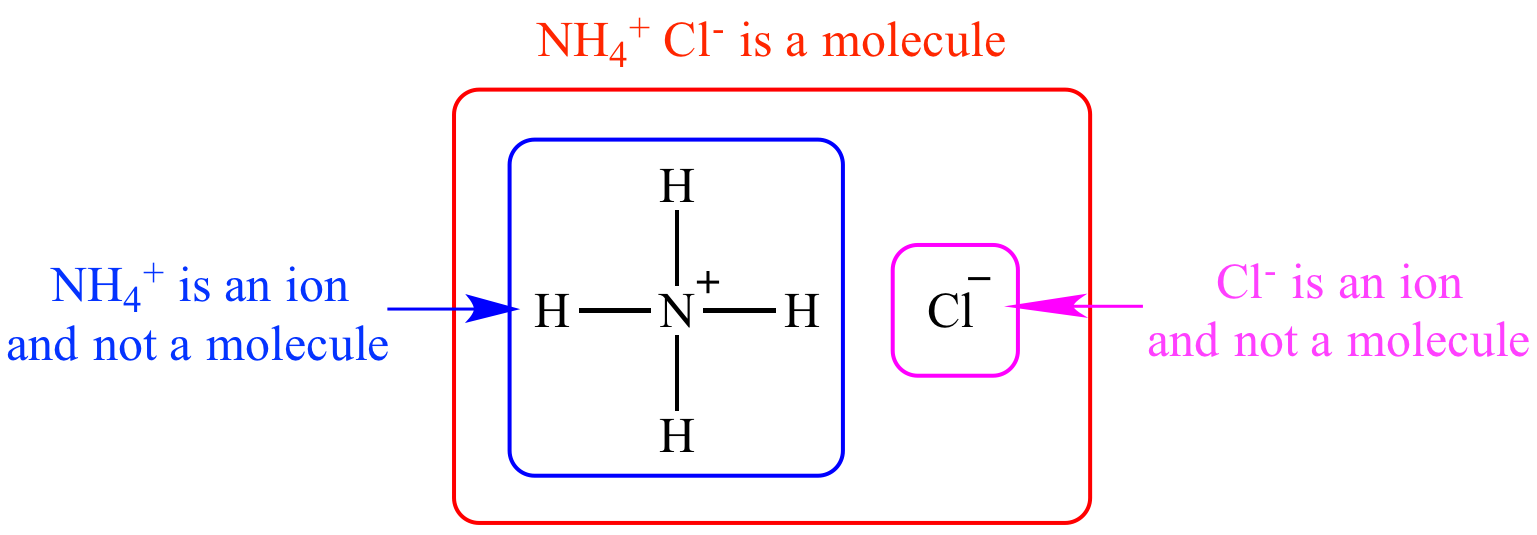
Illustrated Glossary of Organic Chemistry Molecule
Chloride Ion Formula And Charge Let’s take a look at how it is formed. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. The precise pattern depends on the compound. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. sodium chloride, or table salt, is an ionic compound. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. So that the charges are balanced and it is neutral overall. Since the neutral halogen atom. the chloride ion has a single, negative charge. determining ion charges from formula. If given a formula like nacl or feo or fe 2 o 3 you need to be able to calculate the charge of the ions. Let’s take a look at how it is formed.
From www.scribd.com
Common Ions and Their Charges Ion Chloride Chloride Ion Formula And Charge determining ion charges from formula. the chloride ion has a single, negative charge. Since the neutral halogen atom. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. sodium chloride, or table salt, is an ionic compound. So that the charges are balanced and it. Chloride Ion Formula And Charge.
From www.slideserve.com
PPT The Octet Rule PowerPoint Presentation, free download ID2654700 Chloride Ion Formula And Charge determining ion charges from formula. So that the charges are balanced and it is neutral overall. Let’s take a look at how it is formed. Since the neutral halogen atom. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. sodium chloride, or table salt, is. Chloride Ion Formula And Charge.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Chloride Ion Formula And Charge Let’s take a look at how it is formed. Since the neutral halogen atom. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. the chloride ion has a single, negative charge. So that the charges are balanced and it is neutral overall. The precise pattern depends on. Chloride Ion Formula And Charge.
From giojcsdfs.blob.core.windows.net
Chlorine Ionic Bond Form at Janyce Gallegos blog Chloride Ion Formula And Charge The precise pattern depends on the compound. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Since the neutral halogen atom. If given a formula like nacl or feo or fe 2 o 3 you need to be able to calculate the charge of the ions. . Chloride Ion Formula And Charge.
From general.chemistrysteps.com
Writing Chemical Formulas For Ionic Compounds Chemistry Steps Chloride Ion Formula And Charge determining ion charges from formula. the chloride ion has a single, negative charge. Let’s take a look at how it is formed. Since the neutral halogen atom. If given a formula like nacl or feo or fe 2 o 3 you need to be able to calculate the charge of the ions. During the formation of sodium chloride,. Chloride Ion Formula And Charge.
From www.youtube.com
How to Draw the Lewis Dot Structure for Cl (Chloride ion) YouTube Chloride Ion Formula And Charge Since the neutral halogen atom. If given a formula like nacl or feo or fe 2 o 3 you need to be able to calculate the charge of the ions. So that the charges are balanced and it is neutral overall. determining ion charges from formula. The precise pattern depends on the compound. During the formation of sodium chloride,. Chloride Ion Formula And Charge.
From www.slideserve.com
PPT Naming Multivalent Compounds PowerPoint Presentation, free Chloride Ion Formula And Charge sodium chloride, or table salt, is an ionic compound. So that the charges are balanced and it is neutral overall. Since the neutral halogen atom. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. If given a formula like nacl or feo or fe 2 o 3 you. Chloride Ion Formula And Charge.
From slideplayer.com
Chapter 7 Notes Chemical Formulas West Valley High School ppt download Chloride Ion Formula And Charge determining ion charges from formula. sodium chloride, or table salt, is an ionic compound. So that the charges are balanced and it is neutral overall. The precise pattern depends on the compound. Since the neutral halogen atom. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers. Chloride Ion Formula And Charge.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Chloride Ion Formula And Charge chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. Let’s take a look at how it is formed. So that the charges are balanced and it is neutral overall. If given a formula like nacl or feo or fe 2 o 3 you need to be able to. Chloride Ion Formula And Charge.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry Chloride Ion Formula And Charge Let’s take a look at how it is formed. So that the charges are balanced and it is neutral overall. Since the neutral halogen atom. If given a formula like nacl or feo or fe 2 o 3 you need to be able to calculate the charge of the ions. The precise pattern depends on the compound. chloride ion. Chloride Ion Formula And Charge.
From www.slideserve.com
PPT Seawater Chemistry PowerPoint Presentation, free download ID Chloride Ion Formula And Charge Since the neutral halogen atom. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. The precise pattern depends on the compound. determining ion charges from formula. the chloride ion has a single, negative charge. So that the charges are balanced and it is neutral overall. Let’s take. Chloride Ion Formula And Charge.
From slideplayer.com
Chapter 7 Compounds and Their Bonds ppt download Chloride Ion Formula And Charge the chloride ion has a single, negative charge. So that the charges are balanced and it is neutral overall. The precise pattern depends on the compound. Since the neutral halogen atom. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. sodium chloride, or table salt, is an. Chloride Ion Formula And Charge.
From www.slideserve.com
PPT Chapter 9 “Chemical Names and Formulas” PowerPoint Presentation Chloride Ion Formula And Charge So that the charges are balanced and it is neutral overall. Let’s take a look at how it is formed. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Since the neutral halogen atom. The precise pattern depends on the compound. If given a formula like nacl. Chloride Ion Formula And Charge.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chloride Ion Formula And Charge the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. the chloride ion has a single, negative charge. So that the charges are balanced and it is neutral overall. sodium chloride, or table salt, is an ionic compound. chloride ion is a chlorine anion that. Chloride Ion Formula And Charge.
From www.numerade.com
SOLVED Write the formulas for the following ionic compounds in which Chloride Ion Formula And Charge determining ion charges from formula. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Since the neutral halogen atom. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. So that the charges are balanced. Chloride Ion Formula And Charge.
From giowjvcvu.blob.core.windows.net
Chlorine Ion Valence Electrons at John Andrade blog Chloride Ion Formula And Charge the chloride ion has a single, negative charge. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. So that the charges are balanced and it is neutral overall. Since the neutral halogen atom. If given a formula like nacl or feo or fe 2 o 3 you need. Chloride Ion Formula And Charge.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4192871 Chloride Ion Formula And Charge the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. determining ion charges from formula. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. During the formation of sodium chloride, the electron given off by. Chloride Ion Formula And Charge.
From exynigviq.blob.core.windows.net
What Is Chlorine Charge at Christine Calhoun blog Chloride Ion Formula And Charge chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. the chloride ion has a single, negative charge. determining ion charges from formula. Let’s take a look at how it is formed. the formula of an ionic compound represents the simplest ratio of the numbers of. Chloride Ion Formula And Charge.
From www.animalia-life.club
Sodium Chloride Lewis Dot Structure Chloride Ion Formula And Charge the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Since the neutral halogen atom. the chloride ion has a single, negative charge. The precise pattern depends on the compound. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming. Chloride Ion Formula And Charge.
From slideplayer.com
Chapter 4 Compounds and Their Bonds ppt download Chloride Ion Formula And Charge Let’s take a look at how it is formed. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. determining ion charges from formula. If given a formula like nacl or feo or fe 2 o 3 you need to be able to calculate the charge of. Chloride Ion Formula And Charge.
From www.youtube.com
ClO2 Lewis Structure How to Draw the Lewis Structure for ClO2 YouTube Chloride Ion Formula And Charge During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. Since the neutral halogen atom. determining ion charges from formula. the chloride ion has a single, negative. Chloride Ion Formula And Charge.
From www.youtube.com
ClO Lewis Structure How to Draw the Lewis Structure for ClO YouTube Chloride Ion Formula And Charge chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. Let’s take a look at how it is formed. determining ion charges from formula. The precise pattern depends on the compound. So that the charges are balanced and it is neutral overall. Since the neutral halogen atom. If. Chloride Ion Formula And Charge.
From www.shutterstock.com
Vector Illustration Sodium Chloride Formation By Stock Vector (Royalty Chloride Ion Formula And Charge Let’s take a look at how it is formed. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. the chloride ion has a single, negative charge. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of.. Chloride Ion Formula And Charge.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Molecule Chloride Ion Formula And Charge The precise pattern depends on the compound. the chloride ion has a single, negative charge. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. So that the charges are balanced and it is neutral overall. sodium chloride, or table salt, is an ionic compound. Since the. Chloride Ion Formula And Charge.
From slideplayer.com
Ionic Compounds Naming and Writing Formulas ppt download Chloride Ion Formula And Charge If given a formula like nacl or feo or fe 2 o 3 you need to be able to calculate the charge of the ions. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. the chloride ion has a single, negative charge. determining ion charges. Chloride Ion Formula And Charge.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chloride Ion Formula And Charge the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. sodium chloride, or table salt, is an ionic compound. determining ion charges from formula. Since. Chloride Ion Formula And Charge.
From www.numerade.com
SOLVEDThe designations IA through 8 A used for certain families of the Chloride Ion Formula And Charge the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. sodium chloride, or table salt, is an ionic compound. determining ion charges from formula. If given a formula like nacl or feo or fe 2 o 3 you need to be able to calculate the charge. Chloride Ion Formula And Charge.
From www.alamy.com
Sodium Chloride ionic bond formation. NaCl structure. Sodium and Chloride Ion Formula And Charge the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. Since the neutral halogen atom. Let’s take a look at how it is formed. determining ion charges from formula. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium. Chloride Ion Formula And Charge.
From mavink.com
Sodium Chloride Structure Chloride Ion Formula And Charge chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. sodium chloride, or table salt, is an ionic compound. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. the formula of an ionic compound represents the. Chloride Ion Formula And Charge.
From www.pinterest.com.au
Ion Names, Formulas and Charges Chart for Chemistry Classroom Chloride Ion Formula And Charge The precise pattern depends on the compound. determining ion charges from formula. sodium chloride, or table salt, is an ionic compound. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. the formula of an ionic compound represents the simplest ratio of the numbers of ions. Chloride Ion Formula And Charge.
From www.numerade.com
SOLVED Draw a Lewis diagram to show the formation of the ionic Chloride Ion Formula And Charge Let’s take a look at how it is formed. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. sodium chloride, or table salt, is an ionic compound. If given a formula like nacl or feo or fe 2 o 3 you need to be able to. Chloride Ion Formula And Charge.
From gioipnbxg.blob.core.windows.net
Element Chlorine Bonding at Jean Shinn blog Chloride Ion Formula And Charge chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. During the formation of sodium chloride, the electron given off by sodium is taken by chlorine, forming the chloride ion. determining ion charges from formula. the chloride ion has a single, negative charge. If given a formula. Chloride Ion Formula And Charge.
From ar.inspiredpencil.com
Chloride Ion Charge Chloride Ion Formula And Charge Since the neutral halogen atom. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. the chloride ion has a single, negative charge. sodium chloride, or table salt, is an ionic compound. The precise pattern depends on the compound. During the formation of sodium chloride, the electron. Chloride Ion Formula And Charge.
From www.youtube.com
How to Calculate the Formal Charges for ClO2 (Chlorite ion) YouTube Chloride Ion Formula And Charge sodium chloride, or table salt, is an ionic compound. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. So that the charges are balanced and it is neutral overall. If given a formula like nacl or feo or fe 2 o 3 you need to be. Chloride Ion Formula And Charge.
From saylordotorg.github.io
Ionic Bonding and Simple Ionic Compounds Chloride Ion Formula And Charge So that the charges are balanced and it is neutral overall. determining ion charges from formula. the chloride ion has a single, negative charge. chloride ion is a chlorine anion that forms the negatively charged part of certain salts, including sodium and hydrogen chloride. The precise pattern depends on the compound. Since the neutral halogen atom. Let’s. Chloride Ion Formula And Charge.