What Are Gmp Standards . the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice.
from www.vrogue.co
good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in.
What Are Gmp Guidelines Good Manufacturing Practices vrogue.co
What Are Gmp Standards Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice.
From solutionpharmacy.in
GMP Requirements Solution Parmacy What Are Gmp Standards Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products.. What Are Gmp Standards.
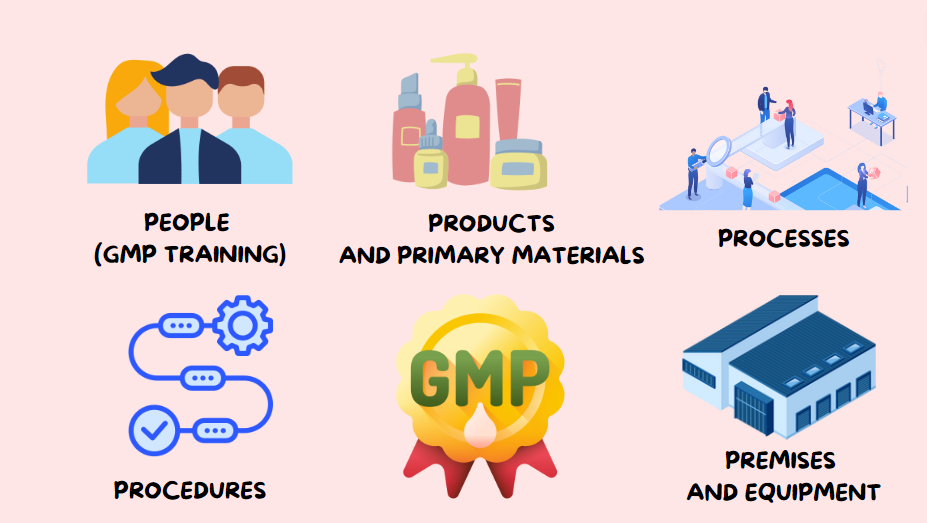
From www.scilife.io
5 Essential Components of GMP A Comprehensive Guide What Are Gmp Standards good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. the main regulatory standard for ensuring pharmaceutical quality is the current good. What Are Gmp Standards.
From www.vrogue.co
What Are Gmp Guidelines Good Manufacturing Practices vrogue.co What Are Gmp Standards good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. the main regulatory standard for ensuring pharmaceutical quality. What Are Gmp Standards.
From inspeedglobal.com
What is GMP and How to Get It? What Are Gmp Standards good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy. What Are Gmp Standards.
From qvalon.com
The Complete Guide to Good Manufacturing Practices (GMP) by QVALON QVALON What Are Gmp Standards good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. the cgmp regulations for. What Are Gmp Standards.
From hoangsame.com
WHAT IS GMP? STANDARDS GMP, CGMP, GMP EU, GMP WHO HOANG SA M&E CLEAN What Are Gmp Standards Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices,. What Are Gmp Standards.
From www.slideserve.com
PPT Good Manufacturing Practices Purpose and Principles of GMP What Are Gmp Standards good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices,. What Are Gmp Standards.
From www.pharmaspecialists.com
GMP Guidelines for Pharmaceutical Industry What Are Gmp Standards the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. the cgmp regulations for. What Are Gmp Standards.
From fyoffxobv.blob.core.windows.net
What Is Good Manufacturing Practices at Johnny Conley blog What Are Gmp Standards good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. good manufacturing practices (gmp, also referred. What Are Gmp Standards.
From www.cbmmaryland.com
GMP Guidelines Commercial Building Maintenance, LLC What Are Gmp Standards the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. good manufacturing practice (gmp) guidelines are. What Are Gmp Standards.
From isolocity.com
All About GMP Guidelines Automate Your Compliance Isolocity What Are Gmp Standards good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice'). What Are Gmp Standards.
From qmciso.org
GMP Good Manufacturing Practice Quality Management Certification What Are Gmp Standards the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. good manufacturing practice (gmp) is a system for. What Are Gmp Standards.
From www.youtube.com
What are GMP Guidelines? Good Manufacturing Practices for Food Safety What Are Gmp Standards good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals,. What Are Gmp Standards.
From dhti.vn
What is a GMP certificate? Steps to a GMPcertified What Are Gmp Standards the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. good manufacturing practice (gmp) guidelines are specific regulations established by. What Are Gmp Standards.
From www.slideserve.com
PPT GOOD MANUFACTURING PRACTICE FOR BIOPROCESS ENGINEERING (ERT 421 What Are Gmp Standards good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. the main regulatory standard for ensuring pharmaceutical quality is the current good. What Are Gmp Standards.
From www.slideshare.net
Good manufacturing practice (GMP) What Are Gmp Standards good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. good manufacturing practice (gmp) is a system for. What Are Gmp Standards.
From hoangsame.com
WHAT IS GMP? STANDARDS GMP, CGMP, GMP EU, GMP WHO HOANG SA M&E CLEAN What Are Gmp Standards good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. the main regulatory standard for ensuring pharmaceutical quality. What Are Gmp Standards.
From www.youtube.com
What is GMP? Good Manufacturing Practices in Food Industry What Are Gmp Standards good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. the cgmp regulations for drugs contain minimum requirements for the. What Are Gmp Standards.
From oic.com.vn
APPLYING GMP STANDARDS TO MANAGE THE HEALTH SUPPLEMENT BUSINESS What Are Gmp Standards good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals,. What Are Gmp Standards.
From www.cbmmaryland.com
The 10 Principles of GMP Commercial Building Maintenance What Are Gmp Standards the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing. What Are Gmp Standards.
From www.compliancequest.com
What is GMP Quality? GMP Standards and Regulations What Are Gmp Standards the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals,. What Are Gmp Standards.
From www.uaeiso.org
GMP Certification Check Benefits, Safety & Integrity Ascent What Are Gmp Standards Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products.. What Are Gmp Standards.
From www.vietfil.com
What is GMP standard in pharmaceutical manufacturing What Are Gmp Standards the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. the main regulatory standard for ensuring pharmaceutical quality is the current good. What Are Gmp Standards.
From www.compliancequest.com
What is GMP Quality? GMP Standards and Regulations What Are Gmp Standards the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. the main regulatory standard for ensuring. What Are Gmp Standards.
From marketbusinessnews.com
Good manufacturing practice definition and meaning What Are Gmp Standards good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced. What Are Gmp Standards.
From www.slideserve.com
PPT Good Manufacturing Practices Purpose and Principles of GMP What Are Gmp Standards good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals,. What Are Gmp Standards.
From www.scilife.io
What are the 5 Main Components of GMP? Full definition Scilife What Are Gmp Standards Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products.. What Are Gmp Standards.
From www.slideserve.com
PPT Good Manufacturing Practices Purpose and Principles of GMP What Are Gmp Standards good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug.. What Are Gmp Standards.
From www.vecteezy.com
GMP Good Manufacturing Practice 6 heading of infographic template with What Are Gmp Standards the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. good manufacturing practice (gmp) is a system for ensuring that products are. What Are Gmp Standards.
From www.inmedpharma.com
What are the GMP manufacturing standards for different products? What Are Gmp Standards the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. good manufacturing practice (gmp) is a system for. What Are Gmp Standards.
From www.youtube.com
Good Manufacturing Practices Certification (GMP) Benefits Approval What Are Gmp Standards good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. Gmp refers to the good. What Are Gmp Standards.
From www.scribd.com
57121843 GMP Guidelines Quality Assurance Quality What Are Gmp Standards good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. the cgmp regulations for drugs contain minimum requirements for the methods, facilities, and controls used in. Gmp refers to the good manufacturing practice regulations promulgated by the us food. What Are Gmp Standards.
From www.pharmaaccess.net
The GMP Framework Pharmaaccess What Are Gmp Standards good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. Gmp refers to the good manufacturing practice regulations promulgated by the us food and drug. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice'). What Are Gmp Standards.
From www.slideserve.com
PPT GOOD MANUFACTURING PRACTICES [GMP] PowerPoint Presentation, free What Are Gmp Standards good manufacturing practice (gmp) guidelines are specific regulations established by local and international authorities to ensure the quality, safety, and efficacy of pharmaceuticals, medical devices, and certain food products. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. Gmp refers to the good manufacturing practice regulations promulgated by the. What Are Gmp Standards.
From www.inmedpharma.com
What are the GMP manufacturing standards for different products? What Are Gmp Standards good manufacturing practice (gmp) is a system for ensuring that products are consistently produced and controlled according to quality standards. good manufacturing practices (gmp, also referred to as 'cgmp' or 'current good manufacturing practice') is the aspect of. the main regulatory standard for ensuring pharmaceutical quality is the current good manufacturing practice. Gmp refers to the good. What Are Gmp Standards.