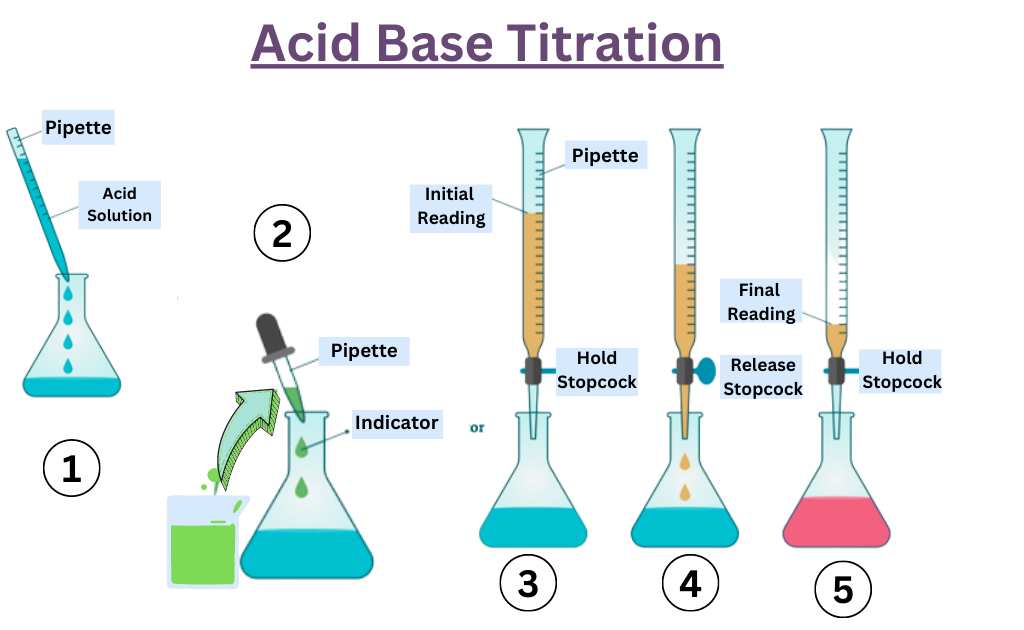
Acid Base Titration Definition Chemistry . The concentration of a solution can be determined by knowing the acid and base dissociation constant. In its simplest form, titration is carried out by measuring the volume of the solution. The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another chemical that changes. The reagent (titrant) is the solution with a known molarity that will react with the analyte. There are two basic types of acid base titrations, indicator and potentiometric.
from themasterchemistry.com
There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. In its simplest form, titration is carried out by measuring the volume of the solution. In an indicator based titration you add another chemical that changes.
Acid Base TitrationWorking Principle, Process, Types And Indicators
Acid Base Titration Definition Chemistry In its simplest form, titration is carried out by measuring the volume of the solution. In its simplest form, titration is carried out by measuring the volume of the solution. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another chemical that changes. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Acid Base Titration Definition Chemistry The concentration of a solution can be determined by knowing the acid and base dissociation constant. In its simplest form, titration is carried out by measuring the volume of the solution. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with. Acid Base Titration Definition Chemistry.
From mavink.com
Acid Base Titration Equation Acid Base Titration Definition Chemistry In an indicator based titration you add another chemical that changes. In its simplest form, titration is carried out by measuring the volume of the solution. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. There are two basic types of acid base. Acid Base Titration Definition Chemistry.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Acid Base Titration Definition Chemistry The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another chemical that changes. There are two basic types of acid base titrations, indicator and potentiometric. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that. Acid Base Titration Definition Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Acid Base Titration Definition Chemistry In an indicator based titration you add another chemical that changes. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the. Acid Base Titration Definition Chemistry.
From www.slideserve.com
PPT Acid Base Titrations PowerPoint Presentation, free download ID Acid Base Titration Definition Chemistry The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you. Acid Base Titration Definition Chemistry.
From mungfali.com
Acid Base Titration Lab Acid Base Titration Definition Chemistry In an indicator based titration you add another chemical that changes. In its simplest form, titration is carried out by measuring the volume of the solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution. Acid Base Titration Definition Chemistry.
From www.savemyexams.com
Core Practical AcidAlkali Titration Edexcel GCSE Chemistry Revision Acid Base Titration Definition Chemistry There are two basic types of acid base titrations, indicator and potentiometric. The reagent (titrant) is the solution with a known molarity that will react with the analyte. In its simplest form, titration is carried out by measuring the volume of the solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. In. Acid Base Titration Definition Chemistry.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Acid Base Titration Definition Chemistry There are two basic types of acid base titrations, indicator and potentiometric. The reagent (titrant) is the solution with a known molarity that will react with the analyte. In an indicator based titration you add another chemical that changes. The analyte (titrand) is the solution with an unknown molarity. In its simplest form, titration is carried out by measuring the. Acid Base Titration Definition Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Acid Base Titration Definition Chemistry In its simplest form, titration is carried out by measuring the volume of the solution. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. There are two basic types of acid base titrations, indicator and potentiometric. The reagent (titrant) is the solution with. Acid Base Titration Definition Chemistry.
From facts.net
8 Captivating Facts About AcidBase Titration Acid Base Titration Definition Chemistry The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. In an indicator based titration you add another chemical that changes. In its simplest form, titration is carried out by measuring the volume of the solution. There are two basic types of acid base. Acid Base Titration Definition Chemistry.
From worksheets.clipart-library.com
AcidBase Titration Definition, Examples, Types, Key Terms Acid Base Titration Definition Chemistry In its simplest form, titration is carried out by measuring the volume of the solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. In an indicator based titration you add another chemical that changes. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte. Acid Base Titration Definition Chemistry.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Acid Base Titration Definition Chemistry In its simplest form, titration is carried out by measuring the volume of the solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. There are two basic types of acid base titrations, indicator and potentiometric. In. Acid Base Titration Definition Chemistry.
From mavink.com
Acid Base Titration Calculation Acid Base Titration Definition Chemistry The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another chemical that changes. The reagent (titrant) is the solution with a known molarity that will react with the analyte. There are two basic types of acid base titrations, indicator and potentiometric. The concentration of a solution can be determined by knowing the. Acid Base Titration Definition Chemistry.
From www.youtube.com
Titration Acid Base Titration Chemistry YouTube Acid Base Titration Definition Chemistry The reagent (titrant) is the solution with a known molarity that will react with the analyte. There are two basic types of acid base titrations, indicator and potentiometric. The concentration of a solution can be determined by knowing the acid and base dissociation constant. In its simplest form, titration is carried out by measuring the volume of the solution. In. Acid Base Titration Definition Chemistry.
From www.chemistrylearner.com
AcidBase Titration Definition, Theory, and Curves Acid Base Titration Definition Chemistry In its simplest form, titration is carried out by measuring the volume of the solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with. Acid Base Titration Definition Chemistry.
From theedge.com.hk
Chemistry How To Titration The Edge Acid Base Titration Definition Chemistry The concentration of a solution can be determined by knowing the acid and base dissociation constant. In its simplest form, titration is carried out by measuring the volume of the solution. In an indicator based titration you add another chemical that changes. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution. Acid Base Titration Definition Chemistry.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Acid Base Titration Definition Chemistry In its simplest form, titration is carried out by measuring the volume of the solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. In an indicator based titration you add another chemical that changes. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte. Acid Base Titration Definition Chemistry.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Acid Base Titration Definition Chemistry There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the. Acid Base Titration Definition Chemistry.
From www.slideserve.com
PPT Acid/Base Titration PowerPoint Presentation, free download ID Acid Base Titration Definition Chemistry The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that will react with the analyte. In its simplest form, titration is carried out by measuring the volume of the solution. In an indicator based titration you add another chemical that changes. There are. Acid Base Titration Definition Chemistry.
From mungfali.com
Acid Base Titration Calculation Acid Base Titration Definition Chemistry There are two basic types of acid base titrations, indicator and potentiometric. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. In its simplest form, titration is. Acid Base Titration Definition Chemistry.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Acid Base Titration Definition Chemistry There are two basic types of acid base titrations, indicator and potentiometric. The reagent (titrant) is the solution with a known molarity that will react with the analyte. In its simplest form, titration is carried out by measuring the volume of the solution. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be. Acid Base Titration Definition Chemistry.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Acid Base Titration Definition Chemistry In its simplest form, titration is carried out by measuring the volume of the solution. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another chemical that changes. There are two basic types of acid base. Acid Base Titration Definition Chemistry.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Acid Base Titration Definition Chemistry In its simplest form, titration is carried out by measuring the volume of the solution. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. In an indicator based titration you add. Acid Base Titration Definition Chemistry.
From www.youtube.com
Acid Base Titration Lecture 3 Chemistry Practicals Fsc 1st Year Acid Base Titration Definition Chemistry In its simplest form, titration is carried out by measuring the volume of the solution. In an indicator based titration you add another chemical that changes. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. There are two basic types of acid base. Acid Base Titration Definition Chemistry.
From scienceinfo.com
Acidbase Titration 4 Types, Theory, Working Principle Acid Base Titration Definition Chemistry The reagent (titrant) is the solution with a known molarity that will react with the analyte. The concentration of a solution can be determined by knowing the acid and base dissociation constant. There are two basic types of acid base titrations, indicator and potentiometric. In its simplest form, titration is carried out by measuring the volume of the solution. In. Acid Base Titration Definition Chemistry.
From www.sciencephoto.com
Acidbase titration Stock Image C015/0435 Science Photo Library Acid Base Titration Definition Chemistry The reagent (titrant) is the solution with a known molarity that will react with the analyte. In an indicator based titration you add another chemical that changes. The analyte (titrand) is the solution with an unknown molarity. The concentration of a solution can be determined by knowing the acid and base dissociation constant. In its simplest form, titration is carried. Acid Base Titration Definition Chemistry.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Acid Base Titration Definition Chemistry There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another chemical that changes. The concentration of a solution can be determined by knowing the acid and base dissociation constant. In its simplest form, titration is carried out by measuring the. Acid Base Titration Definition Chemistry.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Acid Base Titration Definition Chemistry The concentration of a solution can be determined by knowing the acid and base dissociation constant. There are two basic types of acid base titrations, indicator and potentiometric. In its simplest form, titration is carried out by measuring the volume of the solution. The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add. Acid Base Titration Definition Chemistry.
From www.youtube.com
Acid Base Titration Experiment Acid base Titration Practical and Acid Base Titration Definition Chemistry The analyte (titrand) is the solution with an unknown molarity. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes. In its simplest form, titration is carried out by measuring the volume of the solution. The concentration of a solution can be determined by knowing the acid. Acid Base Titration Definition Chemistry.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Acid Base Titration Definition Chemistry The concentration of a solution can be determined by knowing the acid and base dissociation constant. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes. In its simplest form, titration is carried out by measuring the volume of the solution. The reagent (titrant) is the solution. Acid Base Titration Definition Chemistry.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry Acid Base Titration Definition Chemistry The concentration of a solution can be determined by knowing the acid and base dissociation constant. In its simplest form, titration is carried out by measuring the volume of the solution. There are two basic types of acid base titrations, indicator and potentiometric. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with. Acid Base Titration Definition Chemistry.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Acid Base Titration Definition Chemistry The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. In its simplest form, titration is carried out by measuring the volume of the solution. In an indicator based titration you add another chemical that changes. There are two basic types of acid base. Acid Base Titration Definition Chemistry.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Acid Base Titration Definition Chemistry The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another chemical that changes. There are two basic types of acid base titrations, indicator and potentiometric. The concentration of a solution can be determined by knowing the. Acid Base Titration Definition Chemistry.
From stock.adobe.com
Acid base titration experiment and phases of color change during Acid Base Titration Definition Chemistry The analyte (titrand) is the solution with an unknown molarity. In an indicator based titration you add another chemical that changes. There are two basic types of acid base titrations, indicator and potentiometric. The concentration of a solution can be determined by knowing the acid and base dissociation constant. The reagent (titrant) is the solution with a known molarity that. Acid Base Titration Definition Chemistry.
From mungfali.com
Acid Base Titration Calculation Acid Base Titration Definition Chemistry The concentration of a solution can be determined by knowing the acid and base dissociation constant. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the. Acid Base Titration Definition Chemistry.