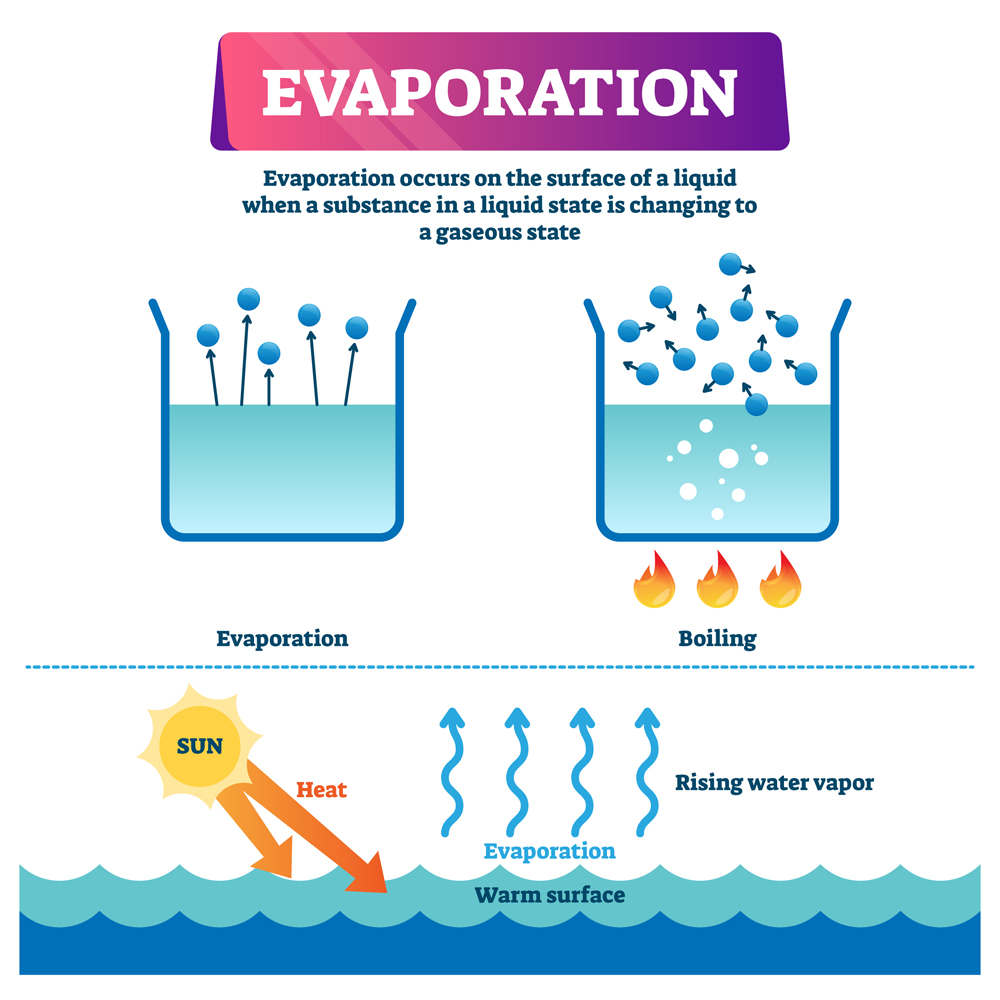
Will Water Evaporate At All Temperatures . When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to. Boiling point of water is 100 degree celsius. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Then how it possible for water to evaporate. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. The temperature at which water in liquid form is converted into gaseous form. When liquid water meets dry air, it is not in equilibrium; Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. The level of humidity in the air also plays a role in the process of evaporation. Calculate how quickly a body of water will evaporate given wind speed and humidity using the evaporation rate calculator.
from www.scienceabc.com
Boiling point of water is 100 degree celsius. The temperature at which water in liquid form is converted into gaseous form. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. When liquid water meets dry air, it is not in equilibrium; When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to. Then how it possible for water to evaporate. Calculate how quickly a body of water will evaporate given wind speed and humidity using the evaporation rate calculator. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk.
Why Does Water Evaporate At Room Temperature?
Will Water Evaporate At All Temperatures When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to. The temperature at which water in liquid form is converted into gaseous form. When liquid water meets dry air, it is not in equilibrium; Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. Calculate how quickly a body of water will evaporate given wind speed and humidity using the evaporation rate calculator. Then how it possible for water to evaporate. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Boiling point of water is 100 degree celsius. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to. The level of humidity in the air also plays a role in the process of evaporation.
From slideplayer.com
Changes in State (PHASE CHANGES)—Notes ppt download Will Water Evaporate At All Temperatures When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to. When liquid water meets dry air, it is not in equilibrium; Then how it possible for water to evaporate. The level of humidity in the air also plays a. Will Water Evaporate At All Temperatures.
From www.slideserve.com
PPT Freezing, Melting, and Evaporation PowerPoint Presentation, free Will Water Evaporate At All Temperatures When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to. The temperature at which water in liquid form is converted into gaseous form. Then how it possible for water to evaporate. Boiling point of water is 100 degree celsius.. Will Water Evaporate At All Temperatures.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Will Water Evaporate At All Temperatures Then how it possible for water to evaporate. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. When liquid water meets dry air, it is not in equilibrium; The level of humidity in the air also plays a role in the process of evaporation. When water temperature reaches 100 ∘c 100 ∘. Will Water Evaporate At All Temperatures.
From www.slideserve.com
PPT Temperature PowerPoint Presentation, free download ID5759542 Will Water Evaporate At All Temperatures The temperature at which water in liquid form is converted into gaseous form. Then how it possible for water to evaporate. The level of humidity in the air also plays a role in the process of evaporation. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Boiling. Will Water Evaporate At All Temperatures.
From slideplayer.com
Intro to Earth’s waters ppt download Will Water Evaporate At All Temperatures Boiling point of water is 100 degree celsius. When liquid water meets dry air, it is not in equilibrium; Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Then how it possible for water to evaporate. Liquid water is made up of molecules of h 2 o. Will Water Evaporate At All Temperatures.
From mavink.com
Water Evaporation Chart Will Water Evaporate At All Temperatures If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. The temperature at which water in liquid form is converted into gaseous form. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Calculate how quickly a body of water will. Will Water Evaporate At All Temperatures.
From vt.audubon.org
The Water Cycle Revisited! Audubon Vermont Will Water Evaporate At All Temperatures Calculate how quickly a body of water will evaporate given wind speed and humidity using the evaporation rate calculator. The level of humidity in the air also plays a role in the process of evaporation. When liquid water meets dry air, it is not in equilibrium; Then how it possible for water to evaporate. Boiling point of water is 100. Will Water Evaporate At All Temperatures.
From www.amazon.ca
Why Does Water Evaporate? All about Heat and Temperature Moore, Rob Will Water Evaporate At All Temperatures Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Then how it possible for water to evaporate. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. The level of humidity in the air also plays a. Will Water Evaporate At All Temperatures.
From www.teachoo.com
Why is Water liquid at room temperature? Give reasons Teachoo Will Water Evaporate At All Temperatures Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Calculate. Will Water Evaporate At All Temperatures.
From www.slideserve.com
PPT The Water Cycle PowerPoint Presentation, free download ID2244191 Will Water Evaporate At All Temperatures Calculate how quickly a body of water will evaporate given wind speed and humidity using the evaporation rate calculator. Then how it possible for water to evaporate. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When water temperature reaches 100 ∘c 100 ∘ c, the molecules. Will Water Evaporate At All Temperatures.
From www.slideserve.com
PPT WATER CYCLE AND WEATHER PowerPoint Presentation, free download Will Water Evaporate At All Temperatures If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. The temperature at which water in liquid form is converted into gaseous form. When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the. Will Water Evaporate At All Temperatures.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Will Water Evaporate At All Temperatures If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. Then how it possible for water to evaporate. When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to. The temperature. Will Water Evaporate At All Temperatures.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Will Water Evaporate At All Temperatures Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces. Will Water Evaporate At All Temperatures.
From www.youtube.com
Heat needed to evaporate only ½ the water Thermodynamics YouTube Will Water Evaporate At All Temperatures Boiling point of water is 100 degree celsius. When liquid water meets dry air, it is not in equilibrium; Then how it possible for water to evaporate. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. The temperature at which water in liquid form is converted into. Will Water Evaporate At All Temperatures.
From www.slideshare.net
Chemistry Chapter 2 matter and change Will Water Evaporate At All Temperatures Then how it possible for water to evaporate. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. The temperature at which water in liquid form is converted into gaseous form. When liquid water meets dry air, it is not in equilibrium; When water temperature reaches 100 ∘c 100 ∘ c,. Will Water Evaporate At All Temperatures.
From tipseri.com
How long does it take for water to evaporate at room temperature? Tipseri Will Water Evaporate At All Temperatures Calculate how quickly a body of water will evaporate given wind speed and humidity using the evaporation rate calculator. Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. Then how. Will Water Evaporate At All Temperatures.
From www.slideserve.com
PPT Freezing, Melting, and Evaporation PowerPoint Presentation, free Will Water Evaporate At All Temperatures When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. When liquid water meets dry air, it is not in equilibrium; Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Then how it possible for water to evaporate. Boiling point. Will Water Evaporate At All Temperatures.
From watermarkexplorer.blogspot.com
Evaporation Water Evaporation Temperature Will Water Evaporate At All Temperatures When liquid water meets dry air, it is not in equilibrium; The level of humidity in the air also plays a role in the process of evaporation. Calculate how quickly a body of water will evaporate given wind speed and humidity using the evaporation rate calculator. Water molecules evaporate off the surface until the amount of water in the air. Will Water Evaporate At All Temperatures.
From www.pxfuel.com
Can Water Evaporate at all Temperatures? Are Evaporation and Boiling Will Water Evaporate At All Temperatures Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the. Will Water Evaporate At All Temperatures.
From 9to5science.com
[Solved] Why does water evaporate at room temperature? 9to5Science Will Water Evaporate At All Temperatures Then how it possible for water to evaporate. When liquid water meets dry air, it is not in equilibrium; Calculate how quickly a body of water will evaporate given wind speed and humidity using the evaporation rate calculator. When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to. Will Water Evaporate At All Temperatures.
From www.youtube.com
Why Does Water Evaporate at Room Temperature? YouTube Will Water Evaporate At All Temperatures The level of humidity in the air also plays a role in the process of evaporation. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. The temperature at which water. Will Water Evaporate At All Temperatures.
From giondslfq.blob.core.windows.net
Will Water Evaporate In The Dark at Victoria Matthes blog Will Water Evaporate At All Temperatures Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. When liquid water meets dry air, it is not in equilibrium; When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. The temperature at which water in liquid form is converted. Will Water Evaporate At All Temperatures.
From www.slideserve.com
PPT Basic Chemistry PowerPoint Presentation, free download ID2465261 Will Water Evaporate At All Temperatures The level of humidity in the air also plays a role in the process of evaporation. Boiling point of water is 100 degree celsius. When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to. Then how it possible for. Will Water Evaporate At All Temperatures.
From studylibrarywulf.z19.web.core.windows.net
Water Pressure Temperature Chart Will Water Evaporate At All Temperatures The temperature at which water in liquid form is converted into gaseous form. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Calculate how quickly a body of. Will Water Evaporate At All Temperatures.
From shinestarpk.blogspot.com
Education World What Is The Evaporation? Will Water Evaporate At All Temperatures Then how it possible for water to evaporate. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts. Will Water Evaporate At All Temperatures.
From www.numerade.com
SOLVEDIf you place water at room temperature in a wellinsulated cup Will Water Evaporate At All Temperatures Water molecules evaporate off the surface until the amount of water in the air creates enough vapour pressure to achieve. Then how it possible for water to evaporate. When liquid water meets dry air, it is not in equilibrium; Boiling point of water is 100 degree celsius. Calculate how quickly a body of water will evaporate given wind speed and. Will Water Evaporate At All Temperatures.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? Will Water Evaporate At All Temperatures Then how it possible for water to evaporate. The level of humidity in the air also plays a role in the process of evaporation. When liquid water meets dry air, it is not in equilibrium; Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When water temperature. Will Water Evaporate At All Temperatures.
From www.metoffice.gov.uk
The water cycle Met Office Will Water Evaporate At All Temperatures When liquid water meets dry air, it is not in equilibrium; The level of humidity in the air also plays a role in the process of evaporation. The temperature at which water in liquid form is converted into gaseous form. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as. Will Water Evaporate At All Temperatures.
From www.youtube.com
How does water evaporate at room temperaturevaporization and Will Water Evaporate At All Temperatures When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to. Boiling point of water is 100 degree celsius. The temperature at which water in liquid form is converted into gaseous form. When the surface is exposed to sunlight, some. Will Water Evaporate At All Temperatures.
From www.researchgate.net
The temperatures of the outlet cooling water at different evaporation Will Water Evaporate At All Temperatures If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. The level of humidity in the air also plays a role in the process of evaporation. Calculate how quickly a body of water will evaporate given wind speed and humidity using the evaporation rate calculator. Then how it possible for water. Will Water Evaporate At All Temperatures.
From how-things-work-science-projects.com
How do Temperature and Wind affect Evaporation? How Things Work Will Water Evaporate At All Temperatures When liquid water meets dry air, it is not in equilibrium; Calculate how quickly a body of water will evaporate given wind speed and humidity using the evaporation rate calculator. Then how it possible for water to evaporate. The level of humidity in the air also plays a role in the process of evaporation. When the surface is exposed to. Will Water Evaporate At All Temperatures.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Will Water Evaporate At All Temperatures When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. The level of humidity in the air also plays a role in the process of evaporation. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. Liquid water is made up of molecules of. Will Water Evaporate At All Temperatures.
From www.slideserve.com
PPT Thermoregulation PowerPoint Presentation, free download ID2602700 Will Water Evaporate At All Temperatures If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. When liquid water meets dry air, it is not in equilibrium; Calculate how quickly a body of water will evaporate given wind speed and humidity using the evaporation rate calculator. Water evaporates at room temperature because the molecules at the surface. Will Water Evaporate At All Temperatures.
From www.youtube.com
Will water evaporate at 50 degrees Celsius? YouTube Will Water Evaporate At All Temperatures Boiling point of water is 100 degree celsius. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into. When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to. When liquid. Will Water Evaporate At All Temperatures.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Evaporation Rate By Temperature Will Water Evaporate At All Temperatures When water temperature reaches 100 ∘c 100 ∘ c, the molecules get so excited that the hydrogen atoms lose the bonds to the oxygen atom and therefore the water starts to. Boiling point of water is 100 degree celsius. Then how it possible for water to evaporate. The level of humidity in the air also plays a role in the. Will Water Evaporate At All Temperatures.