Cup Calorimeter Chemistry . Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. As such, the heat that is measured in such a device is equivalent to the change in. The cup is partially filled with. A coffee cup calorimeter is a constant pressure calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12).
from learningmagicriegel.z4.web.core.windows.net
Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. The cup is partially filled with. As such, the heat that is measured in such a device is equivalent to the change in. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Compare and contrast coffee cup calorimetry and bomb calorimetry. A coffee cup calorimeter is a constant pressure calorimeter. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid.
Coffee Cup Calorimetry Equation
Cup Calorimeter Chemistry Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. The cup is partially filled with. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. As such, the heat that is measured in such a device is equivalent to the change in. Compare and contrast coffee cup calorimetry and bomb calorimetry. A coffee cup calorimeter is a constant pressure calorimeter. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry.
From exoleilmf.blob.core.windows.net
Calorimetric Power Calculation at Martin Stradford blog Cup Calorimeter Chemistry A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A coffee cup calorimeter is a constant pressure calorimeter. Compare and contrast coffee cup. Cup Calorimeter Chemistry.
From users.highland.edu
Calorimetry Cup Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12).. Cup Calorimeter Chemistry.
From www.pearson.com
Chapter 09 16 Constant Pressure Calorimetry (coffee cup) Channels Cup Calorimeter Chemistry A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. Compare and contrast coffee cup calorimetry and bomb calorimetry. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). As such, the heat that is measured in such a device is equivalent to the change in. Calculate. Cup Calorimeter Chemistry.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Cup Calorimeter Chemistry Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. A coffee cup calorimeter is a constant pressure calorimeter. As such, the heat that is measured in such a device is equivalent to the change in. General chemistry students often use simple calorimeters constructed. Cup Calorimeter Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Cup Calorimeter Chemistry Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. As such, the heat that is measured in such a device is equivalent to the change in. Compare and. Cup Calorimeter Chemistry.
From ar.inspiredpencil.com
Coffee Cup Calorimeter Diagram Cup Calorimeter Chemistry General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). Compare and contrast coffee cup calorimetry and bomb calorimetry. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. Using a coffee cup calorimeter,. Cup Calorimeter Chemistry.
From www.thoughtco.com
Coffee Cup and Bomb Calorimetry Cup Calorimeter Chemistry A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. A coffee cup calorimeter is a constant pressure calorimeter. Compare and contrast. Cup Calorimeter Chemistry.
From learningschoolconsoled.z14.web.core.windows.net
Coffee Cup Calorimeter Equation Cup Calorimeter Chemistry As such, the heat that is measured in such a device is equivalent to the change in. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A coffee cup calorimeter is a constant pressure calorimeter. General chemistry students. Cup Calorimeter Chemistry.
From courses.lumenlearning.com
9.2 Calorimetry General College Chemistry I Cup Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The cup is partially filled with. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A coffee cup calorimeter is a constant pressure calorimeter. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in. Cup Calorimeter Chemistry.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Cup Calorimeter Chemistry Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A coffee cup calorimeter is a constant pressure calorimeter. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. Cup Calorimeter Chemistry.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement Cup Calorimeter Chemistry A coffee cup calorimeter is a constant pressure calorimeter. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. A coffee cup calorimeter is. Cup Calorimeter Chemistry.
From learningmagicriegel.z4.web.core.windows.net
Coffee Cup Calorimetry Equation Cup Calorimeter Chemistry A coffee cup calorimeter is a constant pressure calorimeter. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. As such, the heat that is measured. Cup Calorimeter Chemistry.
From www.pinterest.com
ConstantPressure Calorimetery "coffeecup" calorimeter often used to Cup Calorimeter Chemistry A coffee cup calorimeter is a constant pressure calorimeter. As such, the heat that is measured in such a device is equivalent to the change in. Compare and contrast coffee cup calorimetry and bomb calorimetry. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. The cup is partially filled with. Using a coffee cup calorimeter, the. Cup Calorimeter Chemistry.
From www.youtube.com
Coffee Cup Calorimeter Calculate Enthalpy Change, Constant Pressure Cup Calorimeter Chemistry The cup is partially filled with. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring. Cup Calorimeter Chemistry.
From www.shutterstock.com
Coffee Cup Calorimetry Setup Chemistry Diagram Stock Illustration Cup Calorimeter Chemistry A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. As such, the heat that is measured in such a device is equivalent to the change in. The cup is partially filled with. Compare and contrast coffee. Cup Calorimeter Chemistry.
From loejdmoqi.blob.core.windows.net
Calorimeter And The Function at Judith Stanton blog Cup Calorimeter Chemistry A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. A coffee cup calorimeter is. Cup Calorimeter Chemistry.
From joiddcrwa.blob.core.windows.net
What Is Calorimetry Example at Donovan blog Cup Calorimeter Chemistry A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. As such, the heat that is measured in such a device is equivalent to the change in. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances. Cup Calorimeter Chemistry.
From www.pinterest.com
calorimeter cup and beaker (With images) Chemistry lab equipment Cup Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. The cup is partially filled with. As. Cup Calorimeter Chemistry.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Cup Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). As such, the heat that is measured in such a device is equivalent to the change in. The cup is partially filled with. A simple coffee cup calorimeter measures. Cup Calorimeter Chemistry.
From lessonlangdonprowl.z21.web.core.windows.net
Coffee Cup Calorimetry Equation Cup Calorimeter Chemistry General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). The cup is partially filled with. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in. Cup Calorimeter Chemistry.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Cup Calorimeter Chemistry Compare and contrast coffee cup calorimetry and bomb calorimetry. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A coffee cup calorimeter is a constant pressure calorimeter. General chemistry students often. Cup Calorimeter Chemistry.
From ddscalorimeters.com
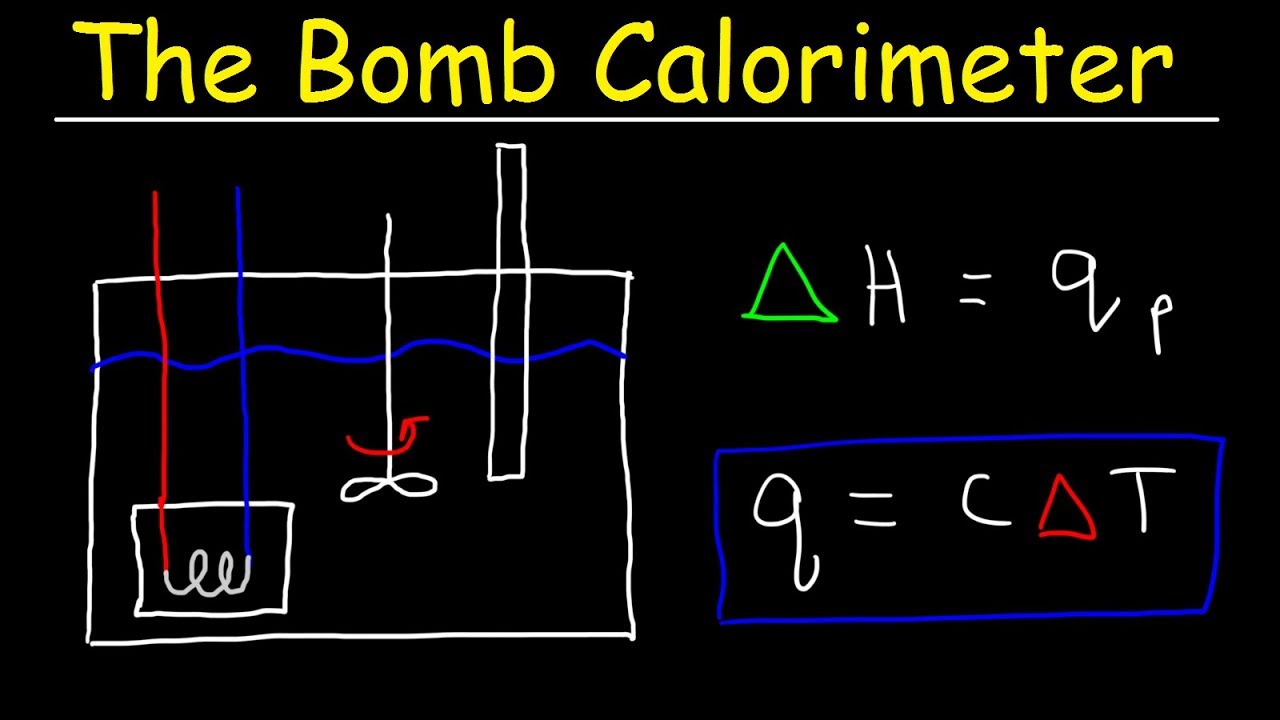
A Coffee Cup Calorimeter vs A Bomb Calorimeter DDS Cup Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. The cup is partially filled with. Compare and contrast coffee cup calorimetry and bomb calorimetry. A coffee cup calorimeter is a constant pressure calorimeter. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. A simple coffee cup calorimeter. Cup Calorimeter Chemistry.
From youtube.com
Chem161 Coffee Cup Calorimetry 6.5 YouTube Cup Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A coffee cup calorimeter is a constant pressure calorimeter. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. The cup is partially filled with. As such, the heat that is measured. Cup Calorimeter Chemistry.
From www.slideserve.com
PPT TOPIC 8 THERMOCHEMISTRY PowerPoint Presentation, free download Cup Calorimeter Chemistry As such, the heat that is measured in such a device is equivalent to the change in. Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances. Cup Calorimeter Chemistry.
From ambrosiabaking.com
Coffee Cup Calorimeter A Simple And Inexpensive Way To Measure The Cup Calorimeter Chemistry A coffee cup calorimeter is a constant pressure calorimeter. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. The cup is partially filled with. As such, the heat that is measured in such a device is equivalent to the change in. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). A simple. Cup Calorimeter Chemistry.
From app.jove.com
Constant Pressure Calorimeter/ Coffee Cup Calorimeter Chemistry JoVe Cup Calorimeter Chemistry As such, the heat that is measured in such a device is equivalent to the change in. The cup is partially filled with. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. A coffee cup calorimeter. Cup Calorimeter Chemistry.
From www.learner.org
The Energy in Chemical Reactions Thermodynamics and Enthalpy Cup Calorimeter Chemistry Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). The cup is partially filled with. Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. A coffee cup calorimeter is essentially a polystyrene. Cup Calorimeter Chemistry.
From learninglibjohn88.z19.web.core.windows.net
Coffee Cup Calorimetry Experiment Cup Calorimeter Chemistry A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. Compare and contrast coffee cup calorimetry and bomb calorimetry. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter.. Cup Calorimeter Chemistry.
From joiddcrwa.blob.core.windows.net
What Is Calorimetry Example at Donovan blog Cup Calorimeter Chemistry General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. Compare and contrast coffee cup calorimetry and bomb calorimetry. Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. The cup is partially filled. Cup Calorimeter Chemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID1552140 Cup Calorimeter Chemistry The cup is partially filled with. A coffee cup calorimeter is a constant pressure calorimeter. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. As such, the heat that is measured in such a device is equivalent to the change in. Compare and. Cup Calorimeter Chemistry.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Cup Calorimeter Chemistry General chemistry students often use simple calorimeters constructed from polystyrene cups (figure 5.12). Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. Calculate the molar heat of enthalpy for a reactions using coffee cup calorimetry. A coffee cup calorimeter is a constant pressure calorimeter. As such, the heat that is measured in such a. Cup Calorimeter Chemistry.
From shaunmwilliams.com
Chapter 6 Presentation Cup Calorimeter Chemistry A coffee cup calorimeter is a constant pressure calorimeter. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. The cup is partially filled with. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached. Cup Calorimeter Chemistry.
From lessonlangdonprowl.z21.web.core.windows.net
Coffee Cup Calorimetry Equation Cup Calorimeter Chemistry Compare and contrast coffee cup calorimetry and bomb calorimetry. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh. Cup Calorimeter Chemistry.
From www.slideserve.com
PPT Chapter 6 THERMOCHEMISTRY PowerPoint Presentation, free download Cup Calorimeter Chemistry A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. Using a coffee cup calorimeter, the heat of neutralization of hcl and naoh is measured. The cup is partially filled with. A coffee cup calorimeter is a constant pressure calorimeter. Calculate heat, temperature change, and specific heat after thermal equilibrium. Cup Calorimeter Chemistry.
From learninglibjohn88.z19.web.core.windows.net
Coffee Cup Calorimeter Equation Cup Calorimeter Chemistry A simple coffee cup calorimeter measures change in enthalpy of a reaction occurring in a solution, under constant pressure conditions. Calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two substances in a calorimeter. A coffee cup calorimeter is essentially a polystyrene (styrofoam) cup with a lid. Using a coffee cup calorimeter, the heat of neutralization. Cup Calorimeter Chemistry.