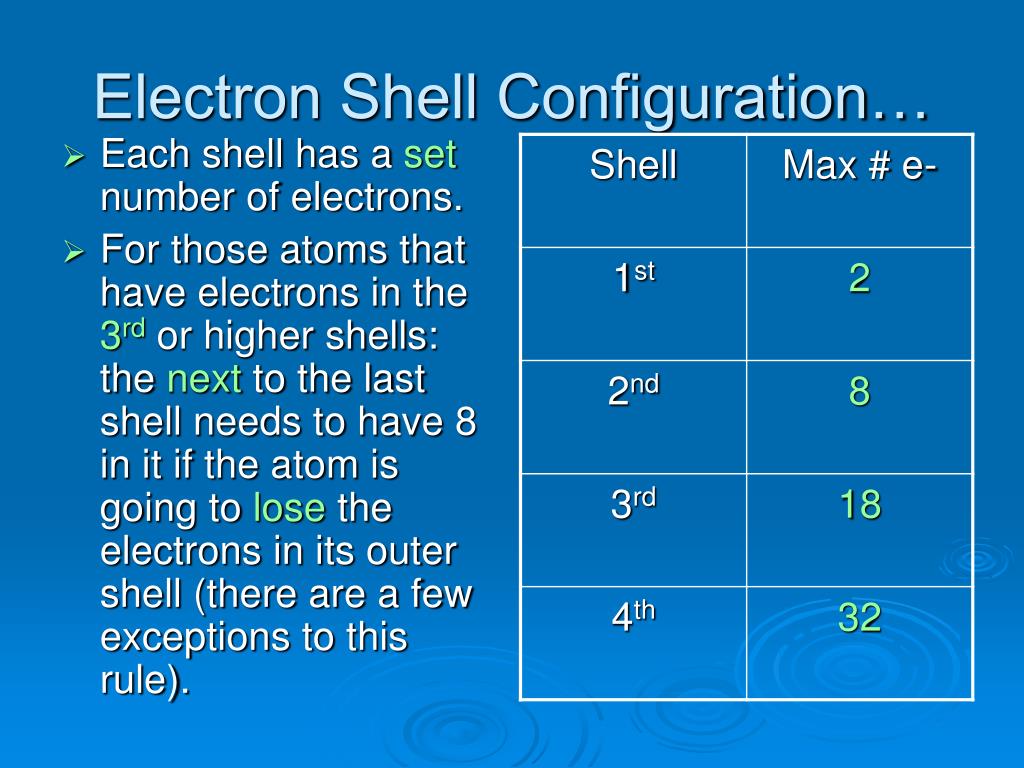
What Does Each Electron Shell Represent . 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements. Learn how many electrons are found in each shell with the rule, order, and. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. And n = 4, 32. The lowest energy level is closest to the nucleus. A full valence shell is the most stable electron configuration. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. Bohr diagrams indicate how many electrons fill each principal shell. The n = 1 electron shell can hold two electrons; Electron shells the energy levels of the electrons within an atom. The editors of encyclopaedia britannica this article. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable. Orbitals most precise information typically available about the energy and. What is an electron shell with a model and diagram.
from www.slideserve.com
And n = 4, 32. A full valence shell is the most stable electron configuration. Bohr diagrams indicate how many electrons fill each principal shell. The lowest energy level is closest to the nucleus. The n = 1 electron shell can hold two electrons; What is an electron shell with a model and diagram. Electron shells the energy levels of the electrons within an atom. 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements. Learn how many electrons are found in each shell with the rule, order, and. Orbitals most precise information typically available about the energy and.
PPT Electron Configuration PowerPoint Presentation, free download
What Does Each Electron Shell Represent Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. Learn how many electrons are found in each shell with the rule, order, and. 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The lowest energy level is closest to the nucleus. And n = 4, 32. A full valence shell is the most stable electron configuration. The editors of encyclopaedia britannica this article. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. Electron shells the energy levels of the electrons within an atom. The n = 1 electron shell can hold two electrons; What is an electron shell with a model and diagram. Orbitals most precise information typically available about the energy and.
From socratic.org
What is the difference between electron shells and electron orbitals What Does Each Electron Shell Represent What is an electron shell with a model and diagram. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable. Bohr diagrams indicate how many electrons fill each principal shell. And n = 4, 32. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons. What Does Each Electron Shell Represent.
From www.breakingatom.com
Electron Configuration and Structure What Does Each Electron Shell Represent The editors of encyclopaedia britannica this article. Learn how many electrons are found in each shell with the rule, order, and. Bohr diagrams indicate how many electrons fill each principal shell. Electron shells the energy levels of the electrons within an atom. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence,. What Does Each Electron Shell Represent.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps What Does Each Electron Shell Represent And n = 4, 32. The lowest energy level is closest to the nucleus. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Learn how many electrons are found in each shell with the rule, order, and. The editors of encyclopaedia britannica this article. The n = 1 electron shell. What Does Each Electron Shell Represent.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry What Does Each Electron Shell Represent Orbitals most precise information typically available about the energy and. The lowest energy level is closest to the nucleus. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. What is an electron shell with a model and diagram. Bohr diagrams indicate how many electrons fill each principal shell.. What Does Each Electron Shell Represent.
From www.thoughtco.com
Electron Configuration Chart What Does Each Electron Shell Represent Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. Learn how many electrons are found in each shell with the rule, order, and. And n = 4, 32. The lowest energy level is closest to the nucleus. 119 rows a useful guide when understanding electron shells in atoms. What Does Each Electron Shell Represent.
From chemistryguru.com.sg
VSEPR Theory and Shapes of Molecules What Does Each Electron Shell Represent Electron shells the energy levels of the electrons within an atom. A full valence shell is the most stable electron configuration. Bohr diagrams indicate how many electrons fill each principal shell. And n = 4, 32. 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements.. What Does Each Electron Shell Represent.
From sciencenotes.org
List of Electron Configurations of Elements What Does Each Electron Shell Represent 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable. Orbitals most precise information typically available about the energy and. Learn how many electrons are found in each. What Does Each Electron Shell Represent.
From courses.lumenlearning.com
Electronic Structure of Atoms (Electron Configurations) Chemistry What Does Each Electron Shell Represent Orbitals most precise information typically available about the energy and. What is an electron shell with a model and diagram. The n = 1 electron shell can hold two electrons; Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Learn how many electrons are found in each shell with the. What Does Each Electron Shell Represent.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID1787201 What Does Each Electron Shell Represent Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. The lowest energy level is closest to the nucleus. 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements. And n = 4, 32. The editors of. What Does Each Electron Shell Represent.
From fity.club
Electron Shell Chemistry And Physics Britannica What Does Each Electron Shell Represent 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements. Learn how many electrons are found in each shell with the rule, order, and. The editors of encyclopaedia britannica this article. The lowest energy level is closest to the nucleus. And n = 4, 32. Group. What Does Each Electron Shell Represent.
From chemistryatomrevision.weebly.com
Electron Configuration Atomic Structure What Does Each Electron Shell Represent The lowest energy level is closest to the nucleus. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. And n = 4, 32. The n = 1 electron shell can hold two. What Does Each Electron Shell Represent.
From courses.lumenlearning.com
Elements and Atoms The Building Blocks of Matter Anatomy and What Does Each Electron Shell Represent And n = 4, 32. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration. The n = 1 electron shell can hold two electrons; Electron shells the energy levels of. What Does Each Electron Shell Represent.
From scientifictutor.org
Chem Bohr Model and Electron Shells Part 1 Scientific Tutor What Does Each Electron Shell Represent Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. 119 rows. What Does Each Electron Shell Represent.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png What Does Each Electron Shell Represent Orbitals most precise information typically available about the energy and. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. And n = 4, 32. The n = 1 electron shell can hold two electrons; Bohr diagrams indicate how many electrons fill each principal shell. A full valence shell is the. What Does Each Electron Shell Represent.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science What Does Each Electron Shell Represent Learn how many electrons are found in each shell with the rule, order, and. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. And n = 4, 32. The editors of encyclopaedia britannica this article. Electron shells the energy levels of the electrons within an atom. What is an electron. What Does Each Electron Shell Represent.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science What Does Each Electron Shell Represent 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Orbitals most precise information typically available about the energy and. Bohr diagrams indicate how many electrons fill. What Does Each Electron Shell Represent.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Does Each Electron Shell Represent The editors of encyclopaedia britannica this article. Learn how many electrons are found in each shell with the rule, order, and. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable. Orbitals most precise information typically available about the energy and. Electron shells the energy levels of the electrons within an atom.. What Does Each Electron Shell Represent.
From fity.club
Electron Shell Chemistry And Physics Britannica What Does Each Electron Shell Represent A full valence shell is the most stable electron configuration. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable. The editors of encyclopaedia britannica this article. Learn how many electrons are found in each shell with the rule, order, and. And n = 4, 32. 119 rows a useful guide when. What Does Each Electron Shell Represent.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho What Does Each Electron Shell Represent Learn how many electrons are found in each shell with the rule, order, and. The n = 1 electron shell can hold two electrons; And n = 4, 32. The editors of encyclopaedia britannica this article. 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements.. What Does Each Electron Shell Represent.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry What Does Each Electron Shell Represent Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. The lowest energy level is closest to the nucleus. A full valence shell is the most stable electron configuration. Learn. What Does Each Electron Shell Represent.
From utedzz.blogspot.com
Periodic Table Electrons Shells Periodic Table Timeline What Does Each Electron Shell Represent Learn how many electrons are found in each shell with the rule, order, and. The n = 1 electron shell can hold two electrons; Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Electron shells the energy levels of the electrons within an atom. Each element’s atom is shown with. What Does Each Electron Shell Represent.
From www.animalia-life.club
Electron Shell Diagram What Does Each Electron Shell Represent What is an electron shell with a model and diagram. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. The n = 1 electron shell can hold two electrons; A full valence shell is the most stable electron configuration. Learn how many electrons are found in each shell. What Does Each Electron Shell Represent.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes What Does Each Electron Shell Represent Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. Orbitals most precise information typically available about the energy and. Learn how many electrons are found in each shell with the rule, order, and. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full. What Does Each Electron Shell Represent.
From www.youtube.com
mr i explains Electron Shells (GCSE Chemistry) YouTube What Does Each Electron Shell Represent Electron shells the energy levels of the electrons within an atom. The editors of encyclopaedia britannica this article. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. Bohr diagrams indicate how many electrons fill each principal shell. The lowest energy level is closest to the nucleus. 119 rows. What Does Each Electron Shell Represent.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Are the Forces That Bind What Does Each Electron Shell Represent Learn how many electrons are found in each shell with the rule, order, and. The editors of encyclopaedia britannica this article. What is an electron shell with a model and diagram. 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements. The lowest energy level is. What Does Each Electron Shell Represent.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free What Does Each Electron Shell Represent Learn how many electrons are found in each shell with the rule, order, and. A full valence shell is the most stable electron configuration. Electron shells the energy levels of the electrons within an atom. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. 119 rows a useful. What Does Each Electron Shell Represent.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Does Each Electron Shell Represent Electron shells the energy levels of the electrons within an atom. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements. A full valence shell. What Does Each Electron Shell Represent.
From www.youtube.com
Electron shells Elements 118 YouTube What Does Each Electron Shell Represent A full valence shell is the most stable electron configuration. The n = 1 electron shell can hold two electrons; What is an electron shell with a model and diagram. Learn how many electrons are found in each shell with the rule, order, and. And n = 4, 32. The editors of encyclopaedia britannica this article. Elements in other groups. What Does Each Electron Shell Represent.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table What Does Each Electron Shell Represent Learn how many electrons are found in each shell with the rule, order, and. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons. What Does Each Electron Shell Represent.
From www.thesciencehive.co.uk
Electron Structure* — the science sauce What Does Each Electron Shell Represent Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Bohr diagrams indicate how many electrons fill each principal shell. Orbitals most precise information typically available about the energy and. Learn how many electrons are found in each shell with the rule, order, and. The editors of encyclopaedia britannica this article.. What Does Each Electron Shell Represent.
From www.slideserve.com
PPT Electron Configuration PowerPoint Presentation, free download What Does Each Electron Shell Represent Orbitals most precise information typically available about the energy and. Learn how many electrons are found in each shell with the rule, order, and. 119 rows a useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements. Group 18 elements (helium, neon, and argon are shown in figure 2). What Does Each Electron Shell Represent.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell What Does Each Electron Shell Represent Learn how many electrons are found in each shell with the rule, order, and. What is an electron shell with a model and diagram. Orbitals most precise information typically available about the energy and. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable. 119 rows a useful guide when understanding electron. What Does Each Electron Shell Represent.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements What Does Each Electron Shell Represent The editors of encyclopaedia britannica this article. Electron shells the energy levels of the electrons within an atom. Group 18 elements (helium, neon, and argon are shown in figure 2) have a full outer, or valence, shell. Each element’s atom is shown with the number of protons in the nucleus with shells of electrons filled by energy levels. Bohr diagrams. What Does Each Electron Shell Represent.
From courses.lumenlearning.com
Electrons Biology for Majors I What Does Each Electron Shell Represent Learn how many electrons are found in each shell with the rule, order, and. Elements in other groups have partially filled valence shells and gain or lose electrons to achieve a stable. And n = 4, 32. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown in figure 2) have. What Does Each Electron Shell Represent.
From www.britannica.com
Electron shell Definition & Facts Britannica What Does Each Electron Shell Represent Orbitals most precise information typically available about the energy and. Bohr diagrams indicate how many electrons fill each principal shell. Learn how many electrons are found in each shell with the rule, order, and. Electron shells the energy levels of the electrons within an atom. Elements in other groups have partially filled valence shells and gain or lose electrons to. What Does Each Electron Shell Represent.