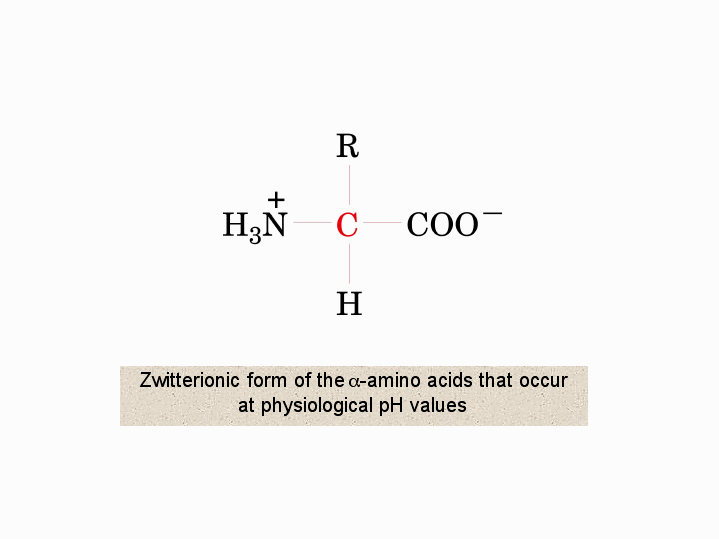Why Are Some Amino Acids Charged At Physiological Ph . the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. in solution, the amino acid molecule appears to have a charge which changes with ph. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. although amino acids can be classified in various ways, one common approach is to classify them according to.
from www3.nd.edu
the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. although amino acids can be classified in various ways, one common approach is to classify them according to. in solution, the amino acid molecule appears to have a charge which changes with ph. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are.
Zwitterionic form of the aamino acids that occur vat physiological pH
Why Are Some Amino Acids Charged At Physiological Ph the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. in solution, the amino acid molecule appears to have a charge which changes with ph. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. although amino acids can be classified in various ways, one common approach is to classify them according to.
From www.drnerz.com
Amino Acid Structures at Physiological pH Why Are Some Amino Acids Charged At Physiological Ph although amino acids can be classified in various ways, one common approach is to classify them according to. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. the weak acid can react with added strong base to form the weak conjugate base,. Why Are Some Amino Acids Charged At Physiological Ph.
From pinbezy.weebly.com
Positively charged amino acids pinbezy Why Are Some Amino Acids Charged At Physiological Ph if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. in the context of amino acids, zwitterions form due to the presence of. Why Are Some Amino Acids Charged At Physiological Ph.
From www.chemistryworld.com
Why are there 20 amino acids? Feature Chemistry World Why Are Some Amino Acids Charged At Physiological Ph in solution, the amino acid molecule appears to have a charge which changes with ph. in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. although amino acids can be classified in various ways, one common approach is to classify them according to. the weak. Why Are Some Amino Acids Charged At Physiological Ph.
From www.slideserve.com
PPT Proteins Structure and Function PowerPoint Presentation, free Why Are Some Amino Acids Charged At Physiological Ph in solution, the amino acid molecule appears to have a charge which changes with ph. in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react. Why Are Some Amino Acids Charged At Physiological Ph.
From mavink.com
Amino Acid Charge Chart Why Are Some Amino Acids Charged At Physiological Ph in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. the isoelectric point (pi) of an amino acid refers to the ph. Why Are Some Amino Acids Charged At Physiological Ph.
From www.slideserve.com
PPT Amino Acids and Peptides PowerPoint Presentation, free download Why Are Some Amino Acids Charged At Physiological Ph although amino acids can be classified in various ways, one common approach is to classify them according to. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+). Why Are Some Amino Acids Charged At Physiological Ph.
From www.coursehero.com
[Solved] What is the net charge of this amino acid at physiological pH Why Are Some Amino Acids Charged At Physiological Ph the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. in solution, the amino acid molecule appears to have a charge which changes with ph. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then. Why Are Some Amino Acids Charged At Physiological Ph.
From www.compoundchem.com
A Brief Guide to the Twenty Common Amino Acids Compound Interest Why Are Some Amino Acids Charged At Physiological Ph the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. although amino acids can be classified in various ways,. Why Are Some Amino Acids Charged At Physiological Ph.
From www.youtube.com
Biochemistry Amino acids Charge on amino acids at specific pH Why Are Some Amino Acids Charged At Physiological Ph in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. although amino acids can be classified in various ways, one common approach is to classify them according to. in solution, the amino acid molecule appears to have a charge which changes with ph. the weak. Why Are Some Amino Acids Charged At Physiological Ph.
From leah4sci.com
Zwitterion and Amino Acid Charge MCAT Biochemistry Tutorial Video Why Are Some Amino Acids Charged At Physiological Ph in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. in solution, the amino acid molecule appears to have a charge which changes with ph. . Why Are Some Amino Acids Charged At Physiological Ph.
From onlinesciencenotes.com
Amino acids General properties and classification Online Science Notes Why Are Some Amino Acids Charged At Physiological Ph if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. although amino acids can be classified in various ways, one common approach is to classify them according to. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in. Why Are Some Amino Acids Charged At Physiological Ph.
From gioxdquht.blob.core.windows.net
Amino Acids Carries A Net Positive Charge At The Physiological Ph at Why Are Some Amino Acids Charged At Physiological Ph in solution, the amino acid molecule appears to have a charge which changes with ph. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. . Why Are Some Amino Acids Charged At Physiological Ph.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Amino Acids Why Are Some Amino Acids Charged At Physiological Ph although amino acids can be classified in various ways, one common approach is to classify them according to. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. the weak acid can react with added strong base to form the weak conjugate base,. Why Are Some Amino Acids Charged At Physiological Ph.
From gioxdquht.blob.core.windows.net
Amino Acids Carries A Net Positive Charge At The Physiological Ph at Why Are Some Amino Acids Charged At Physiological Ph the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. although amino acids can be classified in various ways, one common approach is to classify them according to. if an amino acid has two amino groups and only one carboxyl group (like arginine. Why Are Some Amino Acids Charged At Physiological Ph.
From www3.nd.edu
Zwitterionic form of the aamino acids that occur vat physiological pH Why Are Some Amino Acids Charged At Physiological Ph the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. in solution, the amino acid molecule appears to have a charge which changes with ph. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists. Why Are Some Amino Acids Charged At Physiological Ph.
From www.youtube.com
pH Effects on Amino Acid Structures YouTube Why Are Some Amino Acids Charged At Physiological Ph although amino acids can be classified in various ways, one common approach is to classify them according to. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base. Why Are Some Amino Acids Charged At Physiological Ph.
From www.youtube.com
How to remember the Positively Charge Amino Acids? (aka Basic Amino Why Are Some Amino Acids Charged At Physiological Ph the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. although amino acids can be classified in various ways, one common approach is. Why Are Some Amino Acids Charged At Physiological Ph.
From exywvcvqe.blob.core.windows.net
Amino Acids With Charges at Theresa Randall blog Why Are Some Amino Acids Charged At Physiological Ph the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. in solution, the amino acid molecule appears to have a charge which changes with ph. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists. Why Are Some Amino Acids Charged At Physiological Ph.
From www.slideserve.com
PPT LESSON PLAN PowerPoint Presentation, free download ID9533995 Why Are Some Amino Acids Charged At Physiological Ph in solution, the amino acid molecule appears to have a charge which changes with ph. in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react. Why Are Some Amino Acids Charged At Physiological Ph.
From bradleyabbott.z13.web.core.windows.net
Amino Acid Charge Chart Why Are Some Amino Acids Charged At Physiological Ph the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. in the context of amino acids, zwitterions form due. Why Are Some Amino Acids Charged At Physiological Ph.
From www.drnerz.com
Amino Acid Structures at Physiological pH Why Are Some Amino Acids Charged At Physiological Ph the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. the isoelectric point (pi) of an amino acid refers to the ph at. Why Are Some Amino Acids Charged At Physiological Ph.
From www.slideserve.com
PPT Lecture 2 Eukaryotic cells, amino acids PowerPoint Presentation Why Are Some Amino Acids Charged At Physiological Ph in solution, the amino acid molecule appears to have a charge which changes with ph. in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react. Why Are Some Amino Acids Charged At Physiological Ph.
From www.medschoolcoach.com
Amino Acid Classification MCAT Biochemistry MedSchoolCoach Why Are Some Amino Acids Charged At Physiological Ph in solution, the amino acid molecule appears to have a charge which changes with ph. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then. Why Are Some Amino Acids Charged At Physiological Ph.
From www.slideserve.com
PPT Cell Biology & Molecular Biology of The Cell PowerPoint Why Are Some Amino Acids Charged At Physiological Ph if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. in the context of amino acids, zwitterions form due to the presence of. Why Are Some Amino Acids Charged At Physiological Ph.
From answerhappy.com
Consider the structure of the following charged amino acid, drawn at Why Are Some Amino Acids Charged At Physiological Ph although amino acids can be classified in various ways, one common approach is to classify them according to. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. if an amino acid has two amino groups and only one carboxyl group (like arginine. Why Are Some Amino Acids Charged At Physiological Ph.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master Why Are Some Amino Acids Charged At Physiological Ph in solution, the amino acid molecule appears to have a charge which changes with ph. although amino acids can be classified in various ways, one common approach is to classify them according to. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form.. Why Are Some Amino Acids Charged At Physiological Ph.
From www.youtube.com
69 Structure of basic amino acids at various pH YouTube Why Are Some Amino Acids Charged At Physiological Ph if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. in solution, the amino acid molecule appears to have a charge which changes with ph. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or. Why Are Some Amino Acids Charged At Physiological Ph.
From www.researchgate.net
18 Structure of the amino acids with electrically charged side chains Why Are Some Amino Acids Charged At Physiological Ph in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. in solution, the amino acid molecule appears to have a charge which. Why Are Some Amino Acids Charged At Physiological Ph.
From www.slideserve.com
PPT Ionic Properties of Amino Acids PowerPoint Presentation, free Why Are Some Amino Acids Charged At Physiological Ph in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+) and a. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. in solution, the amino acid molecule appears to have a charge which. Why Are Some Amino Acids Charged At Physiological Ph.
From www.drnerz.com
Amino Acid Structures at Physiological pH Why Are Some Amino Acids Charged At Physiological Ph the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. the weak acid can react with added strong base to form the weak. Why Are Some Amino Acids Charged At Physiological Ph.
From www.gkseries.com
Which out of the following amino acids carries a net positive charge at Why Are Some Amino Acids Charged At Physiological Ph if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists in its neutral, or zwitterionic, form. although amino acids can be classified in various ways, one common approach is. Why Are Some Amino Acids Charged At Physiological Ph.
From www.slideserve.com
PPT Amino Acids & Peptides PowerPoint Presentation, free download Why Are Some Amino Acids Charged At Physiological Ph if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. although amino acids can be classified in various ways, one common approach is to classify them according to. in the context of amino acids, zwitterions form due to the presence of both a protonated amino group (nh3+). Why Are Some Amino Acids Charged At Physiological Ph.
From slidesharenow.blogspot.com
Ph Of Amino Acids Chart slideshare Why Are Some Amino Acids Charged At Physiological Ph in solution, the amino acid molecule appears to have a charge which changes with ph. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. the isoelectric point (pi) of an amino acid refers to the ph at which the amino acid exists. Why Are Some Amino Acids Charged At Physiological Ph.
From www.slideserve.com
PPT Proteins Amino Acid Chains PowerPoint Presentation ID7053765 Why Are Some Amino Acids Charged At Physiological Ph in solution, the amino acid molecule appears to have a charge which changes with ph. although amino acids can be classified in various ways, one common approach is to classify them according to. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong.. Why Are Some Amino Acids Charged At Physiological Ph.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID1205383 Why Are Some Amino Acids Charged At Physiological Ph if an amino acid has two amino groups and only one carboxyl group (like arginine and lysine), then they are. the weak acid can react with added strong base to form the weak conjugate base, and the conjugate base can react with added strong. the isoelectric point (pi) of an amino acid refers to the ph at. Why Are Some Amino Acids Charged At Physiological Ph.