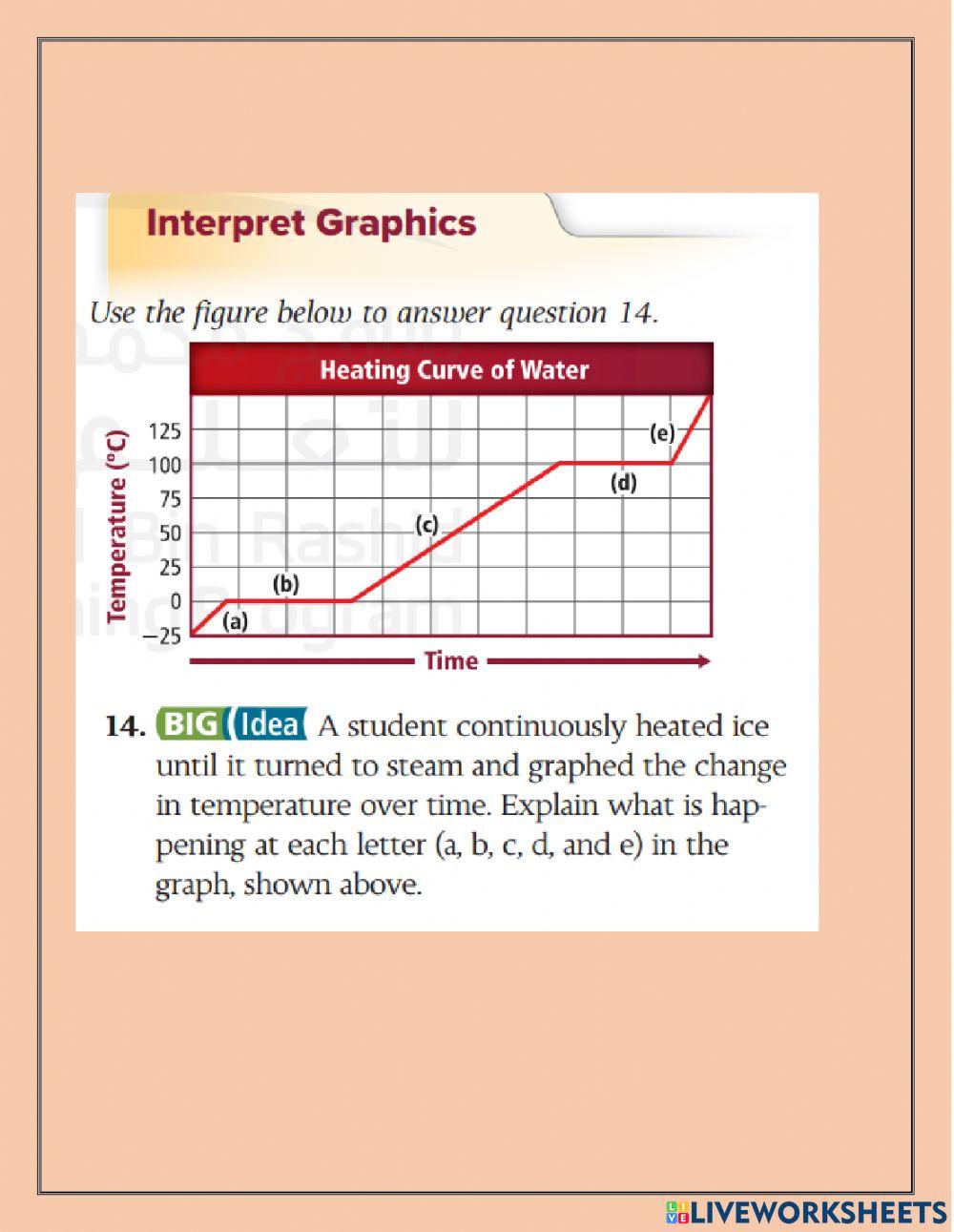
Heating Curve Analysis Worksheet . Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. Working with a partner, sketch a heating curve for water at 101 kpa using the information from the phase diagram. It represents the heating of substance x at a constant rate of. You might think that the temperature goes up. It represents the heating of substance x at a constant rate of heat transfer. Use dimensional analysis or the specific heat equation to complete the following problems. Answer the following using the above heating curve 1. The heating curve shown above is a plot of temperature vs time. Answer the following questions using this. What happens to the temperature of a block of ice when you put a bunsen burner underneath it? Label the melting and boiling point. How much heat is required to melt 25.0 g of ice. 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. What is the melting temperature of the above substance? The heating curve shown above is a plot of temperature vs time.
from www.liveworksheets.com
You might think that the temperature goes up. What happens to the temperature of a block of ice when you put a bunsen burner underneath it? It represents the heating of substance x at a constant rate of. What is the melting temperature of the above substance? The heating curve shown above is a plot of temperature vs time. The heating curve shown above is a plot of temperature vs time. It represents the heating of substance x at a constant rate of heat transfer. Answer the following using the above heating curve 1. Label the melting and boiling point. How much heat is required to melt 25.0 g of ice.
Heating curve 0552 kh Live Worksheets
Heating Curve Analysis Worksheet What happens to the temperature of a block of ice when you put a bunsen burner underneath it? What is the melting temperature of the above substance? You might think that the temperature goes up. Answer the following using the above heating curve 1. 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. It represents the heating of substance x at a constant rate of heat transfer. Use dimensional analysis or the specific heat equation to complete the following problems. What happens to the temperature of a block of ice when you put a bunsen burner underneath it? Answer the following questions using this. It represents the heating of substance x at a constant rate of. Label the melting and boiling point. The heating curve shown above is a plot of temperature vs time. Working with a partner, sketch a heating curve for water at 101 kpa using the information from the phase diagram. How much heat is required to melt 25.0 g of ice. The heating curve shown above is a plot of temperature vs time.
From heatinggondon.blogspot.com
Heating Heating Curve Worksheet Heating Curve Analysis Worksheet 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. Working with a partner, sketch a heating curve for water at 101 kpa using the information from the phase diagram. How much heat is required to melt 25.0 g of ice. What happens to. Heating Curve Analysis Worksheet.
From www.worksheetsgo.com
Heating Curve Worksheets WorksheetsGO Heating Curve Analysis Worksheet 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. How much heat is required to melt 25.0 g of ice. You might think that the temperature goes up. The heating curve shown above is a plot of temperature vs time. It represents the. Heating Curve Analysis Worksheet.
From dokumen.tips
(PDF) Unit 3 Worksheet 2 Heating/Cooling Curve and Energyluckyscience Heating Curve Analysis Worksheet Label the melting and boiling point. How much heat is required to melt 25.0 g of ice. What is the melting temperature of the above substance? The heating curve shown above is a plot of temperature vs time. It represents the heating of substance x at a constant rate of heat transfer. It represents the heating of substance x at. Heating Curve Analysis Worksheet.
From www.numerade.com
SOLVED Emily Jhygeithi VII Heating Curves and Calorimetry Worksheet Heating Curve Analysis Worksheet Use dimensional analysis or the specific heat equation to complete the following problems. It represents the heating of substance x at a constant rate of. What happens to the temperature of a block of ice when you put a bunsen burner underneath it? 1.the heating curve below represents a sample of a substance starting as a solid below its melting. Heating Curve Analysis Worksheet.
From wordworksheet.com
Heating And Cooling Curves Worksheet Heating Curve Analysis Worksheet 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. The heating curve shown above is a plot of temperature vs time. Working with a partner, sketch a heating curve for water at 101 kpa using the information from the phase diagram. It represents. Heating Curve Analysis Worksheet.
From www.housview.com
Heating A Curve Worksheet Free Worksheets Samples Heating Curve Analysis Worksheet How much heat is required to melt 25.0 g of ice. What happens to the temperature of a block of ice when you put a bunsen burner underneath it? Use dimensional analysis or the specific heat equation to complete the following problems. Label the melting and boiling point. It represents the heating of substance x at a constant rate of. Heating Curve Analysis Worksheet.
From rsheatingsashichin.blogspot.com
RS Heating Heating And Cooling Curves Worksheet Heating Curve Analysis Worksheet 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. How much heat is required to melt 25.0 g of ice. It represents the heating of substance x at a constant rate of. Answer the following using the above heating curve 1. Working with. Heating Curve Analysis Worksheet.
From studyfinder.org
The Ultimate Guide to Understanding Worksheet 1 Heating and Cooling Heating Curve Analysis Worksheet How much heat is required to melt 25.0 g of ice. You might think that the temperature goes up. Use dimensional analysis or the specific heat equation to complete the following problems. Working with a partner, sketch a heating curve for water at 101 kpa using the information from the phase diagram. Answer the following using the above heating curve. Heating Curve Analysis Worksheet.
From www.liveworksheets.com
Heating curve 0552 kh Live Worksheets Heating Curve Analysis Worksheet It represents the heating of substance x at a constant rate of. You might think that the temperature goes up. Label the melting and boiling point. What is the melting temperature of the above substance? Working with a partner, sketch a heating curve for water at 101 kpa using the information from the phase diagram. How much heat is required. Heating Curve Analysis Worksheet.
From lessonfullmattie.z21.web.core.windows.net
Heating Cooling Curves Worksheets Answers Heating Curve Analysis Worksheet What is the melting temperature of the above substance? The heating curve shown above is a plot of temperature vs time. It represents the heating of substance x at a constant rate of heat transfer. Answer the following questions using this. Answer the following using the above heating curve 1. The heating curve shown above is a plot of temperature. Heating Curve Analysis Worksheet.
From worksheetzone.org
Heating Curve Worksheet Energy Worksheet Heating Curve Analysis Worksheet The heating curve shown above is a plot of temperature vs time. What is the melting temperature of the above substance? You might think that the temperature goes up. How much heat is required to melt 25.0 g of ice. Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy. Heating Curve Analysis Worksheet.
From learningschoolandy.z21.web.core.windows.net
Heating And Cooling Curves Worksheet Heating Curve Analysis Worksheet It represents the heating of substance x at a constant rate of. The heating curve shown above is a plot of temperature vs time. How much heat is required to melt 25.0 g of ice. What is the melting temperature of the above substance? Use dimensional analysis or the specific heat equation to complete the following problems. What happens to. Heating Curve Analysis Worksheet.
From obropolox.blogspot.com
43 heating cooling curve worksheet answers Worksheet Resource Heating Curve Analysis Worksheet Use dimensional analysis or the specific heat equation to complete the following problems. The heating curve shown above is a plot of temperature vs time. How much heat is required to melt 25.0 g of ice. It represents the heating of substance x at a constant rate of heat transfer. 1.the heating curve below represents a sample of a substance. Heating Curve Analysis Worksheet.
From studylib.net
heating curve worksheet Heating Curve Analysis Worksheet Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. Use dimensional analysis or the specific heat equation to complete the following problems. It represents the heating of substance x at a constant rate of. You might think that the temperature goes. Heating Curve Analysis Worksheet.
From www.studypool.com
SOLUTION Heating Curve and Potential Energy Worksheet Studypool Heating Curve Analysis Worksheet You might think that the temperature goes up. Label the melting and boiling point. The heating curve shown above is a plot of temperature vs time. The heating curve shown above is a plot of temperature vs time. 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over. Heating Curve Analysis Worksheet.
From chessmuseum.org
50 Heating And Cooling Curve Worksheet Heating Curve Analysis Worksheet You might think that the temperature goes up. Working with a partner, sketch a heating curve for water at 101 kpa using the information from the phase diagram. Label the melting and boiling point. What happens to the temperature of a block of ice when you put a bunsen burner underneath it? Use dimensional analysis or the specific heat equation. Heating Curve Analysis Worksheet.
From www.englishworksheet.my.id
Heating And Cooling Curves Worksheet English Worksheet Heating Curve Analysis Worksheet Working with a partner, sketch a heating curve for water at 101 kpa using the information from the phase diagram. You might think that the temperature goes up. Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. It represents the heating. Heating Curve Analysis Worksheet.
From learningcampusstall.z21.web.core.windows.net
Heating And Cooling Curves Worksheet Heating Curve Analysis Worksheet 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. The heating curve shown above is a plot of temperature vs time. Use dimensional analysis or the specific heat equation to complete the following problems. How much heat is required to melt 25.0 g. Heating Curve Analysis Worksheet.
From obropolox.blogspot.com
39 heating cooling curve calculations worksheet answers Worksheet Heating Curve Analysis Worksheet 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. Label the melting and boiling point. The heating curve shown above is a plot of temperature vs time. Use dimensional analysis or the specific heat equation to complete the following problems. It represents the. Heating Curve Analysis Worksheet.
From studytofux1066t.z21.web.core.windows.net
Heating Curve Calculations Worksheets Heating Curve Analysis Worksheet The heating curve shown above is a plot of temperature vs time. Answer the following questions using this. You might think that the temperature goes up. Use dimensional analysis or the specific heat equation to complete the following problems. 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated. Heating Curve Analysis Worksheet.
From studylib.net
Heating Curve Worksheet (1) Heating Curve Analysis Worksheet How much heat is required to melt 25.0 g of ice. Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. The heating curve shown above is a plot of temperature vs time. Label the melting and boiling point. What happens to. Heating Curve Analysis Worksheet.
From studytofux1066t.z21.web.core.windows.net
Heating Curve Calculations Worksheets Heating Curve Analysis Worksheet Answer the following questions using this. What is the melting temperature of the above substance? 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. How much heat is required to melt 25.0 g of ice. Label the melting and boiling point. It represents. Heating Curve Analysis Worksheet.
From www.youtube.com
Unit 6 Heating Curve Worksheet YouTube Heating Curve Analysis Worksheet You might think that the temperature goes up. It represents the heating of substance x at a constant rate of heat transfer. What is the melting temperature of the above substance? Answer the following using the above heating curve 1. 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being. Heating Curve Analysis Worksheet.
From www.onlineworksheet.my.id
Heating Curve Worksheet Answers Onlineworksheet.my.id Heating Curve Analysis Worksheet What is the melting temperature of the above substance? Answer the following questions using this. Answer the following using the above heating curve 1. How much heat is required to melt 25.0 g of ice. What happens to the temperature of a block of ice when you put a bunsen burner underneath it? It represents the heating of substance x. Heating Curve Analysis Worksheet.
From www.uslegalforms.com
Heating Curves Worksheet Fill and Sign Printable Template Online US Heating Curve Analysis Worksheet It represents the heating of substance x at a constant rate of heat transfer. It represents the heating of substance x at a constant rate of. Answer the following using the above heating curve 1. The heating curve shown above is a plot of temperature vs time. Heating curves show that energy is absorbed by a substance as it warms. Heating Curve Analysis Worksheet.
From wordworksheet.com
Heating And Cooling Curves Worksheet Heating Curve Analysis Worksheet Use dimensional analysis or the specific heat equation to complete the following problems. Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. The heating curve shown above is a plot of temperature vs time. What happens to the temperature of a. Heating Curve Analysis Worksheet.
From worksheets.clipart-library.com
Heating Cooling Curve Worksheet Studocu Worksheets Library Heating Curve Analysis Worksheet It represents the heating of substance x at a constant rate of. Use dimensional analysis or the specific heat equation to complete the following problems. Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. What is the melting temperature of the. Heating Curve Analysis Worksheet.
From www.e-streetlight.com
Heating And Cooling Curve Worksheet E Street Light Heating Curve Analysis Worksheet Answer the following using the above heating curve 1. It represents the heating of substance x at a constant rate of heat transfer. Answer the following questions using this. You might think that the temperature goes up. How much heat is required to melt 25.0 g of ice. Heating curves show that energy is absorbed by a substance as it. Heating Curve Analysis Worksheet.
From studylib.net
heating curve worksheet Heating Curve Analysis Worksheet What is the melting temperature of the above substance? 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. Answer the following questions using this. The heating curve shown above is a plot of temperature vs time. The heating curve shown above is a. Heating Curve Analysis Worksheet.
From www.scienceworksheets.net
Heating Curve Worksheet Nahs Physical Science Heating Curve Analysis Worksheet 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. What happens to the temperature of a block of ice when you put a bunsen burner underneath it? Answer the following using the above heating curve 1. Working with a partner, sketch a heating. Heating Curve Analysis Worksheet.
From www.scribd.com
Heating Curve of Water Worksheet Phase (Matter) Phase Transition Heating Curve Analysis Worksheet Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. Label the melting and boiling point. Working with a partner, sketch a heating curve for water at 101 kpa using the information from the phase diagram. It represents the heating of substance. Heating Curve Analysis Worksheet.
From www.e-streetlight.com
Heating And Cooling Curve Worksheet E Street Light Heating Curve Analysis Worksheet 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. Answer the following questions using this. What is the melting temperature of the above substance? Label the melting and boiling point. The heating curve shown above is a plot of temperature vs time. The. Heating Curve Analysis Worksheet.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curve Analysis Worksheet You might think that the temperature goes up. It represents the heating of substance x at a constant rate of heat transfer. 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated over a period of time. Answer the following using the above heating curve 1. It represents the. Heating Curve Analysis Worksheet.
From worksheetzone.org
Free Heating Curve Worksheet Answers For Teaching & Learning Heating Curve Analysis Worksheet Answer the following using the above heating curve 1. Heating curves show that energy is absorbed by a substance as it warms up, melts or boils and that energy is released from a substance as it cools. Working with a partner, sketch a heating curve for water at 101 kpa using the information from the phase diagram. The heating curve. Heating Curve Analysis Worksheet.
From davida.davivienda.com
Heating Curve Worksheet With Answers Printable Word Searches Heating Curve Analysis Worksheet It represents the heating of substance x at a constant rate of heat transfer. Use dimensional analysis or the specific heat equation to complete the following problems. The heating curve shown above is a plot of temperature vs time. 1.the heating curve below represents a sample of a substance starting as a solid below its melting point and being heated. Heating Curve Analysis Worksheet.